The splitting apart of breaking up of U-235 is defined as what?
Nuclear Fission
What are the 3 types of radiation that we talked about in class?
Alpha, beta, gamma
What is something that is always emitted in alpha radiation?
A helium nucleus or an alpha particle
What is one beneficial use of radiation?
Radiation therapy, radiotracing, carbon dating
What is the atomic number of the new isotope produced after radon undergoes alpha decay?
84
Two atoms that combine together and release a ton of energy in the process is defined as what?
Nuclear Fusion
List the types of decay in order of penetrating power (greatest to least).
Gamma, beta, alpha
What is an alternative name for a beta particle?
An electron
What device is used to detect radiation?
What is the mass number of Uranium-235 after it undergoes alpha decay?
231
Both Fission and Fusion reactions release massive amounts of what in addition to their respective atomic nuclei?
Energy
What is a radioisotope?
What is the charge of an alpha particle?
+2
Name both units that are used in determining radiation exposure
What two things are produced if carbon-14 undergoes beta decay?
Nitrogen-14 and an electron
What subatomic particle is primarily responsible for causing chain reactions involved in fission?
Neutrons!
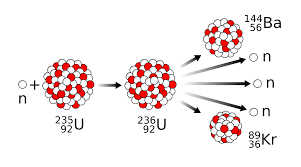
In chemical reactions, electrons are responsible for the new products. What subatomic particles are at play in nuclear reactions?
All of them. Protons, neutrons, and electrons.
What happens to the mass number in beta radiation? What happens to the atomic number in beta radiation?
Stays the same. Goes up by one.
What makes it so cancer patients immune systems get compromised?
The decrease in white blood cell population caused by radiation.
What changes about an atom if it is only undergoing gamma decay?
Nothing. Gamma rays have no charge or mass.
What is one advantage that fusion has over fission?
Fusion products are not radioactive OR lightweight isotopes are abundant in nature
Compare the strengths of the strong force and the electromagnetic force in an element that is actively undergoing radioactive decay.
Electromagnetic force > strong force when something is decaying.
Describe why in alpha radiation the mass number decreases by 4 and the atomic number decreases by 2
2 protons + 2 neutrons = Change in mass of 4
2 protons = Change in atomic number of 2
What does exposure to radiation produce that is the reason that it is harmful?
Free radicals.
Explain why in beta radiation the mass number changes by 0 and the atomic number increases by 1
Neutron gets changed into a proton and an electron. Proton mass = neutron mass so it doesn't change, but atomic number does change because protons increased.