These three particles are used to create an atom
What are protons, neutrons, and electrons?
This is the FIRST element on the periodic table, with an atomic number of 1!
What is Hydrogen?

This is the magic number that ALL atoms want to have on their outer-most (valence) orbit
What is 8?
This classification of matter (of which an electron is one) cannot be broken down into smaller bits
What are Leptons?
This element is famously radioactive, and is used in most modern nuclear fission reactors
What is Uranium?

Part of an atom that is very tiny, and has a negative charge
What is an electron?
This is the number of neutrons in Boron
What is 6?
When combined with two Hydrogen atoms, this element creates water!
What is Oxygen?
This sub-type of Hadrons is made of three quarks bonded together
What are Baryons?
This famous equation, discovered by Einstein, allows us to predict the amount released when a mass is converted directly into energy

What is E=mc2?
This determines the TYPE of element an atom is
What is the # of protons?
"Aurum" in Latin, this element normally has 118 neutrons.
What is Gold?

Table salt is a combination of these two elements.

What is Sodium and Chlorine?
This type of matter is formed when you combine a down quark and an anti-down quark
What is a Meson?
This is the nuclear process that releases a tremendous amount of energy by combining two smaller elements to form one larger one.
What is fusion?
You can determine this by subtracting the atomic mass by the atomic number
What is the # of neutrons?
This element with 30 neutrons is found in everything from steel constructions to your blood!
What is Iron?
One Calcium atom would bond easily with one atom of this element that normally has 16 neutrons.
What is Sulfur?
These are the six types of quarks
What are up, down, top, bottom, strange, and charm?
This is how long the nuclear waste from a fission reaction will take to decay.
What is 10,000+ years?
This is the force that binds the protons and neutrons together in the nucleus of an atom
What is the strong force?
This element, often found in bananas, will bond VERY easily with Chlorine & Fluorine.
What is Potassium?

This family of elements is too "high and mighty" to bond with any other elements, since it already has a complete outer shell of electrons.
What are Noble Gases?

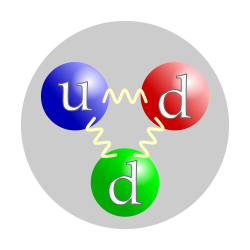
This is how to create a neutron using quarks.
What are a down quark, an up quark, and another down quark?
These are where all the elements other than hydrogen were created, the only place where fusion naturally occurs in the universe.
What are stars?
