What is the stereochemistry of Hydroboration oxidation and Oxymercuration oxidation addition?
No Bonus points but I'll think you're really cool if you can name the components of each reaction
Hydroboration: Syn
BH3 H2O2 NaOH
Oxymercuration Reduction: Anti
Hg(OAC)2 H2O/ NaBH4 NaOH
What Does the n represent in Huckel's rule?
Huckel's rule
4n+2=
The equation represents the number of pi electrons in a molecule and if n is a whole number then the molecule is aromatic
What are the reactants in the halogenation of benzene and what is the mechanism
Bonus points: is the addition of another substituent going to be ortho, meta, or para
(can be more than one)
X-X + FeX3 X= Cl or Bromine
Bonus points: ortho or para
(weakly meta deactivating)
What are the products of their reactions? Thermo or kinetic product? Faster or slower? More stable or less stable?
1.
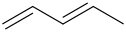 HBr
HBr
----------->
Temp>40* c
2.
 HBr
HBr
------------>
Temp<0* c
1. Thermo Product, faster, more stable
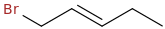
2. Kinetic product, slower, less stable
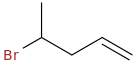
What's my product?
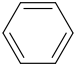 +
+ 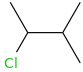 AlCl3
AlCl3
-------->
Alkylation carbocation can rearrange thus this is the product.
-benzene.png)
Which of the 2 Diels alder Reaction dienes would proceed faster
Assume Cis stereochemistry, with the software I'm using I can't make it cis, use your imagination.
A.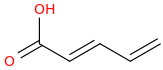
or
B.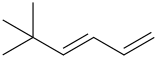
The carboxylic acid is electron-withdrawing and thus decreases the reactivity of the pi bonds needed for the reaction to take place. Therefore
Option B is faster
Am I Aromatic? Why?
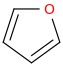
2 double bonds and 1 lone pair means 6 pi electrons, the conjugated double bonds keep it planar, and the molecule can resonate. Therefore Aromatic
What are the reactants in Sulfonation and Nitration and what are both of their mechanisms
Bonus points: is the addition of another substituent going to be ortho, meta, or para
(can be more than one)
Sulfonation SO3 +H2SO4
Nitration HNO3 +H2SO4
Bonus points:
Both will cause the next addition to be Meta because the positive charge on Nitrogen and partial positive of the Sulfur will push away the carbocation which means meta addition is the most stable.
What's my product?
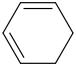 +
+  -------> ?
-------> ?
Endo or Exo?

Neither endo or exo, substituents are planar thus endo or exo cannot be determined
Form the product
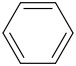 ?
? 
--------->
1. Nitration (causes next additions to be meta)
2. Halogenation
Create a frost circle for Cycloocta-1,3,5,7-tetraene
Answer sheet
Am I Aromatic? Why?

Not planar, 4 double bonds so 8 pi bonds, Cannot Resonate. Anti aromatic
Give the reactants of both Alkylation and Acylation, explain what makes them different, and why it's important to know the distinction
Alkylation: R-X + FeX3 X=Cl or Br
Acylation:
R(with some double bonded substituent )-x +FeX3 X=Cl or Br
Alkylation addition causes the next addition reaction to be either Ortho or Para
Acylation addition may add a substituent that can cause the next addition to prefer meta addition (COOH as an example) over ortho or para so be cautious.
What's my product (this is more of a EAS question but also kinda review)
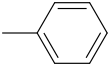 KMNO4 ?
KMNO4 ?
--------->
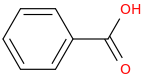
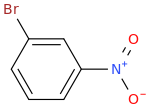
What is my product
 ?
?
1. (Br2 +FeBr3)
2. (HNO3 + H2SO4)
3. (CH3-CH2-Br + AlBr3)
----------------------->
Br is a halogen and thus weakly deactivates the Meta positioning of Nitro. Alkylation can't occur on Nitrobenzene thus.
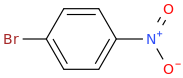 Para
Para
or
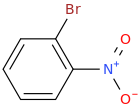 Ortho
Ortho
Name all of the Benzene Derivatives (i will give partial credit)
1. 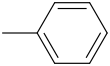 2.
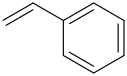
2.
3.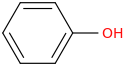 4.
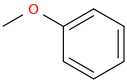
4. 
5.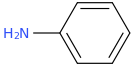 6.
6.
1.Toulene (Methylbenzene)
2.styrene (Vinylbenzene)
3. Phenol (Benzenol) (Fe!nol if you're Travis Scott)
4. Anisole (Methoxybenzene)
5. Aniline (Aminobenzene)
6. Benzoic Acid
Am I Aromatic? Why?
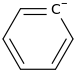
3 double bonds and one lone pair that isn't in the plane of the other pi bonds thus doesn't matter. same as benzene aromatic
Classify each of the substituents and determine if they are Ortho/Para deactivating or Meta Deactivating
(ie where would the next addition go?)
Substituents:
-OH,-Cl,-NO2,-HSO3,-NH2,-CH2Br,-OCOOH,-COOH
Ortho/Para directing:
-OH,-Cl(slightly),-NH2,-OCOOH,-CH2Br(slightly)
Meta Directing:
-NO2,-HSO3,-COOH
What's my product?
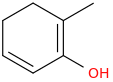 --------->
--------->-4%2C5%2C6-trimethylbicyclo%5B2.2.2%5D-3-octanone.png)
(there is another minor product with same reaction but this is the major product)
1. Diels alder with (z)buta-2-ene
2. tautomerization
(occurs spontaneously for stability)
Form the Product
 ?
? 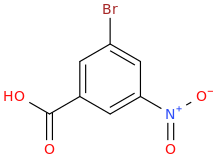
----------->
3. Halogenation
how can you transform an alkyne to a ketone?
Ultimately anything that adds a Alcohol to a alkyne so
Hydroboration Oxidation (With use of DisiamylBorane)
Acid-catalyzed hydration
Oxymercuration oxidation
(editors note: Terminal alkyne creates an aldehyde)
Am i Aromatic >:)
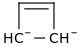
1 double bond and 4 lone pair pi electrons, 6 pi electrons, Conjugated, capable of resonance.
Aromatic
What's wrong with the following synthesis
1. Nitration
2. Alkylation
3. Halogenation
The positive charge on the nitrogen along with the electron-drawing nature of the NO2 group will cause the Benzene to not want to accept the Nv. However Halogenation can still occur because the +charge on the halogen is much smaller, and also because halogens are smaller then the R groups added via Alkylation
Form the Product
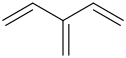 ?
? -3%2C4-dimethylcyclohexane.png)
------------->
1. Diels alder with buta-2-ene
2. 1,4 kinetic rxn of HBr (+Heat)
3. Hydroboration oxidation
4. E2 rxn with TButO- or some other very large strong base for the anti-Zaitsev removal
5. tautomerization will occur naturally for stability
Form the Product.
(1 rule: Any r Groups for alkylation or Acylation CANNOT contain Oxygen)

?
---------->
-2-bromo-4-sulfobenzene.png)
1. Alkylation will
2. Sulfonation
3. Halogenation
RXNS with R group
4. Hydroboration oxidation (Disiamylborane)