In an emergency, an individual with type AB antigen on his red blood cells
- may receive a transfusion of type O blood.
- may receive a transfusion of type A blood.
- may receive a transfusion of type B blood.
- All of the above
- None of the above
- All of the above
What is the best piece of glassware to use to transfer exactly 50 mL of liquid?
- 50 mL beaker
- 50 mL Erlenmeyer flask
- 50 mL volumetric pipet
- 50 mL volumetric flask
- 500 mL graduated cylinder
50 mL volumetric pipet
Which of the following are always considered terminal functional groups?
- Aldehydes
- Ketones
- Carboxylic acids
- Alkenes
- Both A and C
Both A and C
A submarine sends out a sonar signal (sound wave) in a direction directly downward. It takes 2.3 s for the sound wave to travel from the submarine to the ocean bottom and back to the submarine. How high up from the ocean floor is the submarine? (The speed of sound in water is 1,490 m/s.)
- 1,71 m
- 1,715 m
- 1,713.5 m
- 1,713 m
- 11,713.5 m
1,713.5 m
The manufacturer of Sleep-EZ mattresses is offering a 10% discount on the price of its king- size mattress. Some retailers are offering additional discounts. If a retailer offers an additional 20% discount, then what is the total discount available at that retailer?
- 10%
- 25%
- 28%
- 30%
- 32%
28%
Which of the following molecules is thought to have acted as the first enzyme in early life on earth?
- Triglycerides
- DNA
- RNA
- Phospholipids
- Protein
RNA
A sample of argon occupies 50 L at standard temperature. Assuming constant pressure, what volume will the gas occupy if the temperature is doubled
- 25 L
- 50 L
- 100 L
- 200 L
- 250 mL
100L
PV=nRT
Fractional distillation would be best used to separate which of the following compounds?
- Methylene chloride (b.p. 41°C) and water (b.p. 100°C)
- Ethyl acetate (b.p. 77°C) and ethanol (b.p. 80°C)
- Aniline (b.p. 184°C) and benzyl alcohol (b.p. 22°C)
- Aniline (b.p. 184°C) and water (b.p. 100°C)
- Ethyl acetate (b.p. 77°C) and aniline (b.p. 184°C)
Ethyl acetate (b.p. 77°C) and ethanol (b.p. 80°C)
Two billiard balls, one moving at 0.5 m/s and the other at rest, undergo a perfectly elastic collision.
If the masses of the billiard balls are equal and the speed of the stationary one after the collision is 0.5 m/s, then what is the speed of the other ball after the collision?
- 0 m/s
- 0.5 m/s
- 0.1 m/s
- 0.2 m/s
- 1.5 m/s
0 m/s
A crate of apples contains 1 bruised apple for every 30 apples in the crate. Three out of every four bruised apples are considered not fit to sell, and every apple that is not fit to sell is bruised. If there are 12 apples not fit to sell in the crate, then how many total apples are there in the crate?
- 270
- 360
- 480
- 600
- 720
480
In which of the following stages of embryo development are the three primary germ layers first present?
- Blastula
- Morula
- Gastrula
- Coelomate
- Zygote
Gastrula
Which element has the greatest electronegativity?
- Chlorine
- Oxygen
- Sulfur
- Fluorine
- Phosphorus
Fluorine
Which of the following products will be formed if 2- methyl-2-butene is reacted with hot basic KMnO4?
- 1 mole of acetic acid and 1 mole of propanoic acid
- 2 moles of pentanoic acid
- 1 mole of acetic acid and 1 mole of acetone
- 2 moles of acetic acid and 1 mole of CO
- 1 mole of pentanoic acid
1 mole of acetic acid and 1 mole of acetone
A hydraulic press has two pistons. One piston of radius 5 cm pushes down on an enclosed fluid and has a 45 kg weight resting on it. The other, larger piston has a radius of 20 cm. What force is needed on the larger piston to keep the press in equilibrium?
- 1200 N
- 3200 N
- 5200 N
- 7200 N
- 9200 N
F (Force) = P (Pressure) x A (Surface Area)
7200 N
Ron begins reading a book at 4:30 p.m. and reads at a steady pace of 30 pages per hour. Michelle begins reading a copy of the same book at 6:00 p.m. If Michelle started 5 pages behind the page that Ron started on and reads at an average pace of 50 pages per hour, at what time would Ron and Michelle be reading the same page?
- 7:00 p.m.
- 7:30 p.m.
- 8:00 p.m.
- 8:30 p.m.
- 9:00 p.m.
8:30 p.m.
When a population reaches its carrying capacity
- Density-dependent factors no longer play a role
- Density-independent factors no longer play a role
- The population size begins to decrease
- Other populations will be forced out of the habitat
- The population growth rate approaches zero
The population growth rate approaches zero
The half-life of radioactive sodium is 15 hours. How many hours would it take for a 64 g sample to decay to one-eighth of its original activity?
- 3
- 15
- 30
- 45
- 60
45
Mass spectroscopy results in the separation of fragments according to
- atomic mass
- mass-to-charge ratio
- viscosity
- absorption wavelength
- principal quantum number
mass-to-charge ratio
Water flows from a pipe of diameter 0.15 m into one of diameter 0.20 m. If the speed in the 0.15 m pipe is 4.88 m/s, what is the speed in the 0.20 m pipe?
- 0.7 m/s
- 1.7 m/s
- 2.7 m/s
- 7.7 m/s
- 9.7 m/s
A1*V1 = A2*V2
2.7 m/s
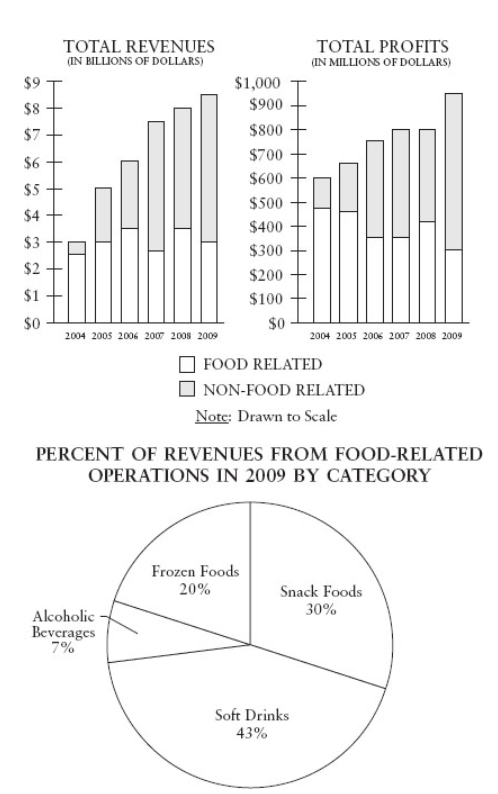
Approximately how much did total revenues increase from 2004 to 2007?
- $0.5 billion
- $1.5 billion
- $4 billion
- $4.5 billion
- $5 billion
$4.5 billion
Which is NOT a type of worm?
- Platyhelminthes
- Nematoda
- Annelida
- Porifera
- None of the above
Porifera
What is the sum of the coefficients of the following equation when it is balanced?
C6 H12 O6 + O2 → CO2 + H2O
- 19
- 20
- 21
- 38
- 40
19
In the ¹³C NMR spectrum of methyl phenyl ketone, which carbon atom will appear the farthest downfield?
- The methyl carbon
- The carbon at the first position of the phenyl ring
- The carbon at the para position of the phenyl ring
- The carbonyl carbon
- The carbon at the meta position of the phenyl ring
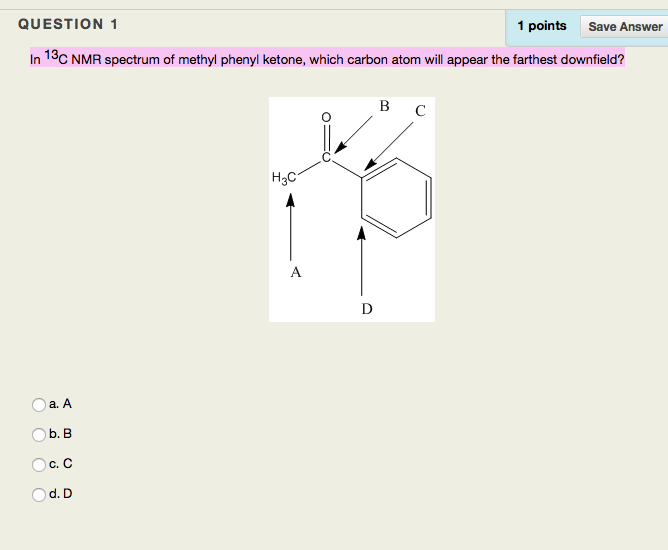
B. The carbonyl carbon
A positive charge of + Q is fixed at point R a distance d away from another positive charge of +2 Q fixed at point S. Point A is located midway between the charges, and point B is a distance d/2 from the +2 Q charge
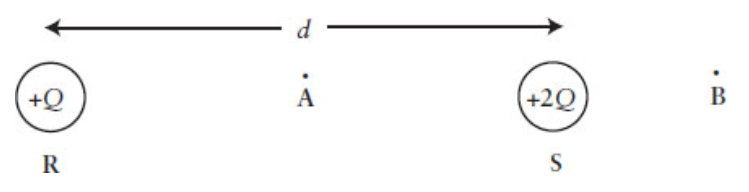
In which direction will a positive charge move if placed at point A?
- Toward the +Q charge.
- Toward the +2Q charge.
- there will be no force
- limited data
- None of the above
Toward the +Q charge.
At an ice cream store, there are 5 flavors of ice cream: strawberry, vanilla, chocolate, mint, and banana. How many different 3-flavor ice cream cones can be made?
- 5
- 30
- 10
- 20
- 60
10
5C3 = 5! / [(5-3)! * 3!]