What type of bonds do alkanes have?
Single bonds
What type of bond do alkenes have?
Double bonds
What type of bonds do alkynes have?
Triple bonds
What atoms make up carboxylic acids
Carbons, Oxygens and Hydrogens
What attaches to the oxygen in an alcohol functional group?
Hydrogen atom
What is the general formula for alkanes
CnH2n+2
What is the general formula of alkenes
CnH2n
What is the general formula for alkynes?
CnH2n-2
What bonds make up carboxylic acids?
Single and double bonds
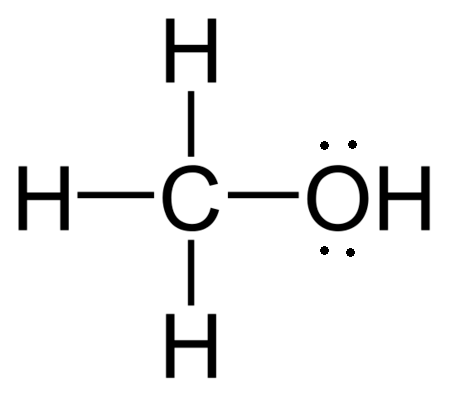
Methanol
Name the following:
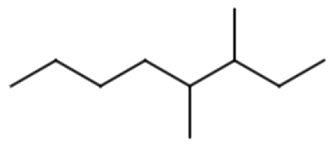
3,4- dimethyloctane
Using the alkene formula. What is the molecular formula when a hydrocarbon has 12 carbons.
24 carbons
How many carbons on the longest chain?
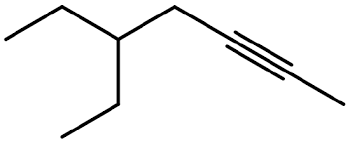
7 carbons
Name the following Carboxylic Acid

Butanoic Acid
a) How many hydroxyl groups does this organic compound have?
b) Which carbon is are these hydroxyl groups coming out of?
a)2
b) 2nd and 4th carbon
When the number of carbons increase, what happens to the boiling point of the hydrocarbon. Explain why.
The boiling point increases, this is because the intermolecular forces become more stronger, and the increase in electrons and surface area increase this.
Name the following:
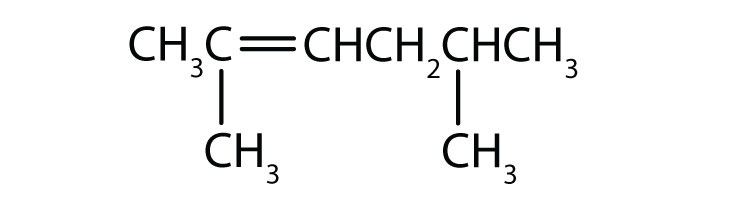
2,5-dimethylhex-2-ene
Use the condensed formula CH3CH2CCCH2CH3 to answer the questions.
a)Draw the structural formula
b) Which carbon is the triple bond coming out of?
a) 
b) 3rd carbon on the longest carbon chain
What is the carboxylic acid functional group called, and what is it made up of?
Carboxyl group (COOH)
Draw the following alcohol 2,3-dimethylpentan-2-ol
Answer on board !!
Use the organic molecule below to answer the questions.
a) Draw the structural formula for this molecule ?
b) What is the IUPAC name for this molecule?
a) Answer on the board !!
b) 3-ethyl-3-methyloctane
What does a double bond in terms of electrons symbolize?
It symbolizes the sharing of 2 electron pairs/6 electrons.
* valence electrons
Draw the skeletal formula for 4,5,5-trimethylhexyne.
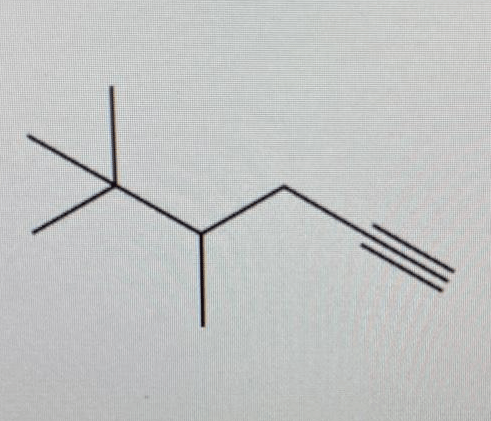
Using the diagram below answer the questions.

a) Name the branched carboxylic acid
b) What type of formula is displayed in the diagram?
a) 2-ethylhexanoic acid
b) Skeletal formula
Using the diagram below answer the questions.
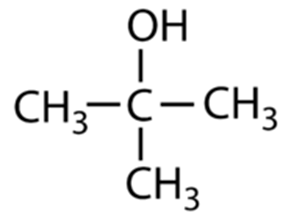
a) What type of alcohol is shown
b) Using IUPAC naming, name the molecule
a) Tertiary Alcohol
b) Propan-2-ol