Which of the following molecules will not have a dipole moment?
A) CH3Cl
B) CH3OCH3
C) CH2Cl2
D) CCl4
D) CCl4
The alkane CH3CH2C(CH3)2CH2CH(CH3)2 has how many 1º, 2º, 3º & 4º carbon atoms?
1º 2º 3º 4º
A)4 2 2 1
B)5 1 2 1
C)5 2 1 1
D)6 1 1 1
C)5 2 1 1
Which of the following statements is not correct?
A) a pair of enantiomeric compounds will have the same melting point.
B) a pair of enantiomeric compounds will have the same solubility in ethanol.
C) a pair of enantiomeric compounds will have exactly the same functional groups.
D) a pair of enantiomeric compounds will have identical optical rotations.
D) a pair of enantiomeric compounds will have identical optical rotations.
Which of the following organic chlorides will not give a Friedel-Craft alkylation product when heated with benzene and AlCl3.
A) (CH3)3CCl
B) CH2=CHCH2Cl
C) CH3CH2Cl
D) CH2=CHCl
D) CH2=CHCl
Which of the following is a correct name for (C2H5)2C=C(CH3)CH2CO2H?
A) 4,4-diethyl-3-methyl-3-butenoic acid
B) 4-ethyl-3-methyl-3-hexenoic acid
C) 3-ethyl-4-methyl-3-hexenoic acid
D) 3-ethyl-4-methyl-3-hexen-6-oic acid
B) 4-ethyl-3-methyl-3-hexenoic acid
How many structurally distinct (different) sets of hydrogens are present in (CH3)3CCH2OCH3?
A) 2
B) 3
C) 4
D) 8
B) 3
A chiral C6H12 hydrocarbon undergoes catalytic hydrogenation to yield an achiral C6H14 product. What is the starting compound?
A) cis-2-hexene
B) 3-methyl-2-pentene
C) 4-methyl-2-pentene
D) 3-methyl-1-pentene
D) 3-methyl-1-pentene
Which of the following statements must be true for two pure chiral isomers ?
A) they must be enantiomers
B) they must be diastereomers
C) they must be stereoisomers
D) they must be optically active
D) they must be optically active
The SN2 reaction of 1-chloro-3-methylbutane with sodium methoxide is relatively slow, but can be accelerated by the addition of a small amount of NaI.
How is this catalysis best explained?
A) The sodium cation helps pull off the chloride anion
B) The iodide anion activates the methoxide nucleophile
C) SN2 reaction of iodide ion converts the alkyl chloride to the more reactive alkyl iodide
D) The NaI changes the mechanism to SN1
C) SN2 reaction of iodide ion converts the alkyl chloride to the more reactive alkyl iodide
Which of the following reagents does not react with benzoic acid, converting it into a different compound?
A) NaI in acetone
B) SOCl2
C) LiAlH4 in ether
D) excess CH3Li in pentane
A) NaI in acetone
Which of the following cations is most stable?
A) FH2+
B) OH3+
C) NH4+
D) CH5+
C) NH4+
What reagents and conditions are used for the Simmons-Smith reaction?
A) CH3I + Mg in ether
B) CH2I2 + Zn (Cu) in ether
C) BrCH2CH2Br + Zn in ether
D) CBr4 + Zn (Cu) in ether
B) CH2I2 + Zn (Cu) in ether
How many stereoisomers of (CH3)2CHCH=CHCH2CH(OH)CH2Br are possible?
A) 2
B) 3
C) 4
D) 5
C) 4
The reaction of sodium ethoxide with iodoethane to form diethyl ether is classified as ...
A) an electrophilic substitution
B) a nucleophilic substitution
C) a radical substitution
D) an electrophilic addition
B) a nucleophilic substitution
A C5H8 hydrocarbon is reacted with BH3 in THF, followed by oxidation with alkaline hydrogen peroxide.
Treatment of the resulting product with PCC in CH2Cl2 produces a chiral ketone, formula C5H8O.
What hydrocarbon best fits these facts?
A) 1-methylcyclobutene
B) methylenecyclobutane
C) vinylcyclopropane
D) cyclopentene
A) 1-methylcyclobutene
The following compounds have similar molecular weights. Which has the highest boiling point?
A) CH3CH=O
B) C2H5OH
C) CH3OCH3
D) CH3CH2CH3
B) C2H5OH
Which of the following reagents would be best for oxidizing a 1º-alcohol to an aldehyde?
A) H3PO4
B) PCC in CH2Cl2
C) Jones' reagent (H2CrO4)
D) OsO4
B) PCC in CH2Cl2
Pure (S)-2-butanol has a specific rotation of +13.52 degrees. You have made and purified a sample that has a calculated specific rotation of +6.76 degrees.
What can you conclude about this sample?
A) the sample has completely racemized
B) 50% the sample has rearranged into a meso isomer
C) 50% of the sample has racemized
D) 75% of the sample has racemized
C) 50% of the sample has racemized
How might one best accomplish the following synthesis?
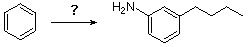
A) (i) C4H9Cl + AlCl3 (ii) HNO3 & heat (iii) excess H2 & Pt catalyst
B) (i) HNO3 & heat (ii) C4H9Cl + AlCl3 (iii) excess H2 & Pt catalyst
C) (i) C3H7COCl + AlCl3 (ii) HNO3 & heat (iii) excess H2 & Pt catalyst
D) (i) HNO3 & heat (ii) C3H7COCl + AlCl3 (iii) excess H2 & Pt catalyst
C) (i) C3H7COCl + AlCl3 (ii) HNO3 & heat (iii) excess H2 & Pt catalyst
Which of the following aldehydes, used alone, will undergo an aldol reaction?
A) formaldehyde, CH2O
B) butanal, CH3(CH2)2CHO
C) benzaldehyde, C6H5CHO
D) 2-propenal, CH2=CHCHO
B) butanal, CH3(CH2)2CHO
Which functional group has a positive and negative formal charge in it and where are they?
Nitro (-NO2)
N+ and one of the O's is O-
Define the difference between a polar protic and a polar aprotic solvent and give an example of each.
Polar Protic: Has Hydrogen bonding. Ex: Water, Alcohols, etc
Polar Aprotic: No hydrogen bonding. Ex: DMSO, DMF, Acetone, etc
Draw (2R, 3S)-2,3-pentanediol?
2nd OH is wedged
3rd OH is dashed
If I react cyclohexanone with NH4Cl and KCN, what would my major product be?
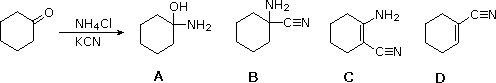
B
Which of the following procedures would not be suitable for preparing 3-methyl-1-phenyl-1-butanone, C6H5COCH2CH(CH3)2?
A) (i) C6H5COCH=CHCH3 + (CH3)2CuLi in ether; (ii) H3O+ workup
B) (i) benzene + (CH3)2CHCH2COCl & AlCl3 (ii) H3O+ workup
C) (i) C6H5MgBr + (CH3)2CHCH2CHO in ether; (ii) H3O+ workup; (iii) PCC in CH2Cl2
D) (i) (CH3)2CHMgBr + C6H5COCH3 in ether; (ii) H3O+ workup; (iii) PCC in CH2Cl2
D) (i) (CH3)2CHMgBr + C6H5COCH3 in ether; (ii) H3O+ workup; (iii) PCC in CH2Cl2