Is the lone pair in this compound localized or delocalized?
Localized because there is no resonance
What is another term for an electrophile?
Lewis Acid
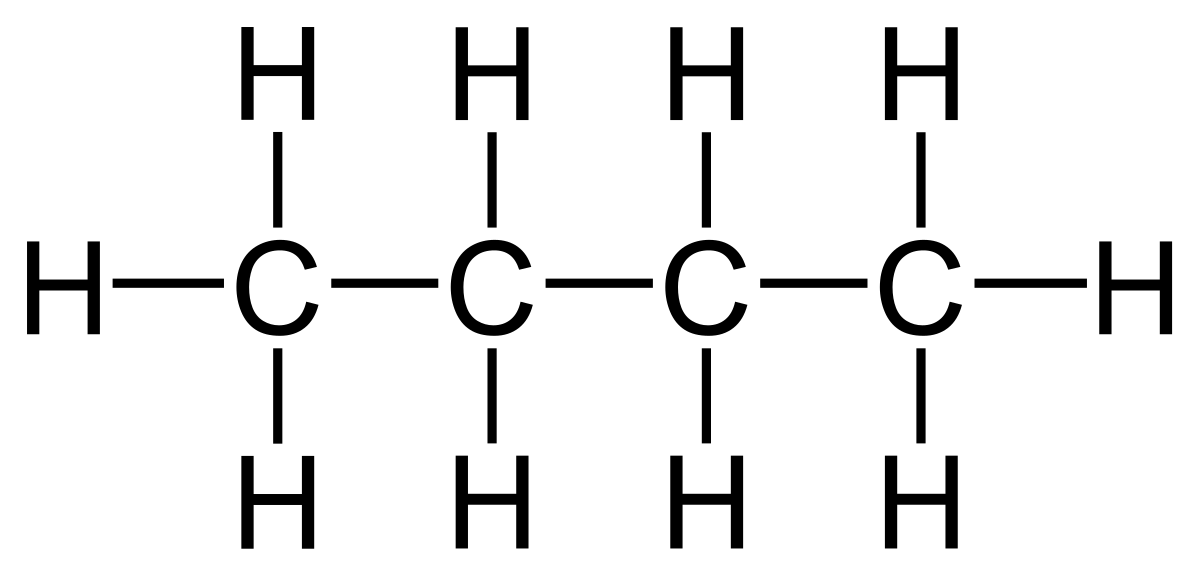
How many methylene groups are present in 2,4-dimethylhexane?
2 methylene groups
Which has a lower energy level, bonding or anti-bonding molecular orbitals?
bonding orbitals have a lower energy level, which makes bonds more favorable energetically
Calculate the hybridization for all atoms in (CH3)3O+
All carbons and the oxygen are sp3 hybridized
What are the 4 rules to consider when determining the major contributor to the resonance structure?
1. fill as many octets as possible
2. make as many bonds as possible
3. the negative charge is on the most electronegative atom
4. as minimal charge separation as possible
Which base is stronger, Cl- or I- ?
Cl- because it is more electronegative
Electronegativity is highest in the upper-right corner of the periodic table
What functional group is contained in CH3CH2OCH2CH3 ?
Ether
What is the difference between atomic orbitals and molecular orbitals?
atomic orbitals represent electrons around a single nucleus
molecular orbitals represent electron distribution in a molecule (2+ nuclei)
Alkanes are hydrocarbons that only contain ___ hybridized carbons
sp3 hybridized
Draw the resonance structures for CH3NO, indicate the major and minor contributor
Drawn on the board
List the following molecules in order of increasing boiling point:
n-hexane
2-methylpentane
2,3-dimethylbutane
2,3-dimethylbutane > 2-methylpentane > n-hexane
Branching decreases boiling point because there is less surface area and therefore less intermolecular attractions (Van der Waals dispersion forces)
Write the condensed formula for this compound:
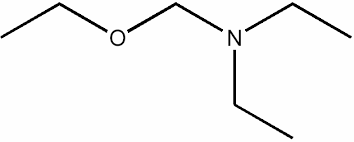
CH3CH2OCH2N(CH2CH3)2
Draw the bonding and anti-bonding pi molecular orbitals for O2
drawn on the board
What is the bond angle of the sp2 hybridized carbon in this compound?
120 degrees
What is the conjugate acid of CH3OH (methanol)?
CH3OH2+
What is the IUPAC name of the compound below?
3-Bromo-4-ethyl-2-methylheptane
Draw the least stable chair confirmation of:
cis-1-isopropyl-4-methylcyclohexane.
Indicate 1,3-diaxial interactions if they exist
(Chair conformation drawn on the board)
Draw two resulting resonance structures of this molecule, there is a + on carbon 1. Indicate the major and minor contributor

drawn on the board
Which compound is most acidic?



This compound is the most acidic, determined by drawing the conjugate base and using ARIO factors. Oxygen is more electronegative than Nitrogen, and the second ring does not exhibit resonance.
Name 4 functional groups that are found in this molecule
Amide
Alcohol
Amine
Aromatic rings
Not: Ketone, aldehyde
Draw the Newman projection for 2,3-dimethylbutane that has 2 Gauche interactions when looking down C2-C3.
What is the torsional energy barrier for this conformation?
E= 3.8kJ/mol + 3.8kJ/mol
E = 7.6 kJ/mol
(Projection drawn on the board)