These compound(s) are not Hydrocarbons:
a. C6H6 b. CO2 c. CH2O d.CaC2 e. CH3NH2
What are:
b. CO2 c. CH2O d.CaC2 e. CH3NH2
This hetroatom compound was first used as a general anesthetic in 1846.
What is ether (diethyl ether)?
This simple triple bonded substance is used to generate temperatures above 3,500 °C (6,330 °F).
What is ethyne (acetylene)?
The simplist branced hydrocarbon:
What is

The number of valence electrons for carbon.
What is 4
A carbon that is double bonded to just one other carbon will have how bonding electrons left to bond with Hydrogen? a. 1 b.2 c. 3 d. 4
What is 2?
This is the way substitutes are listed according to IUPAC.
What is alphabetically
The IUPAC name of this compound
What is 1,2 Dimethylcyclobutane?
The skeletel structure for dipropylamine
What is
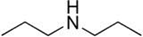
This is the simplist alkyl substitute group.
What is methyl
These compounds have a non alkyl functional group a. pentanol b. 2-methylbutane c. 2,2-dimethylpropane
What is pentanol?
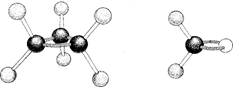
These 3D models are named for the components used to construct them.
What are "ball and stick" models?
These IUPAC compounds respresent the simplist hydrocarbon ring and aldehyde.
What are cyclopropane and methanal (formaldehyde).
2-methyl, 3-hexene can be represented by this structural formula
what is
This structure formula is for pentanoic acid
What is 
This molecule is a. saturated b. non saturated c. branched alkane
CH3(CH2)13CH3
What is saturated?
Bromine, Chlorine, Fluorine, and Iodine will generate these substitues, when bonded to a carbon atom:
What are bromo, chloro, fluoro, and Iodo?
The IUPAC name for this compound
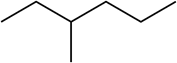
What is 3-methylhexane
The structural formula of 2-Bromo, 1,3-Chloropropane
What is
1,2-dibromo-2-methylbutane is an example of this.
What is an Alkyl halide (haloalkane)
This type bond will limit Carbon to one bonding electron
a. single b. double c. triple d. covalent e. ionic
What is a triple bond?
This organization developed a set of rules which are used to name organic compounds (the acronym is NOT good enough).
What is the International Union of Pure and Applied Chemistry?
The IUPAC name for this compound
What is cyclohexylamine?
This is the skeletal structure for a carboxylic acid containing 5 carbons in a chain.
What is

The theory that organic compounds can only be made by living organisms:
What is vitalism