How many moles of CaCO3 are there in a 3.05g sample?
What is 0.0305mol?
Name this compound.
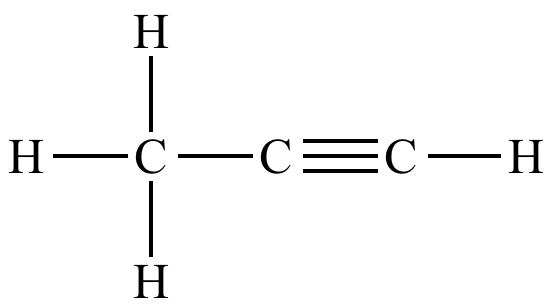
What is propyne?
This process takes longer/larger hydrocarbon compounds and breaks them into multiple smaller compounds.
What is cracking?
Balance the following half-reaction:
Cl2 --> 2Cl-
What is Cl2 + 2e- --> 2Cl-
State if the reaction between tin solution and a piece of cadmium is spontaneous or non-spontaneous.
What is a spontaneous reaction?
A hair product requires you to combine 20.0mL of hydrogen peroxide with enough water to produce a solution with a total volume of 120.0mL. Determine the percent by volume concentration of the solution.
What is 16.7%?
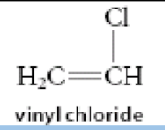
Draw the repeating polymer unit for Vinyl chloride.
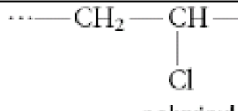
Draw 2,5-dimethyl-3-hexyne in a line diagram.
Identify the oxidizing agent and reducing agent in this reaction.
AgNO3 + Cu --> Cu(NO3)2 + Ag
Oxidizing agent: Ag+(aq)
Reducing agent: Cu(s)
This process takes crude oil and heats it up to separate out the hydrocarbons by size.
What is fractional distillation?
In a chemical analysis of 500mL of carbonated water, 0.36g of carbon dioxide was measured. What is the concentration of carbon dioxide in parts per million?
What is 720ppm?
Draw 4-ethyl-2,3-dimethylheptane.
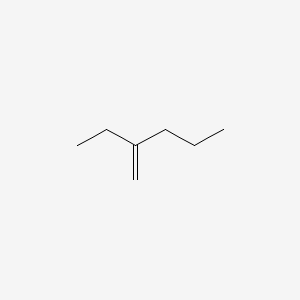
Name the following compound:
What is 2-ethyl-1-pentene?
State if the reaction between Hg(l) and Chromium solution is spontaneous or non-spontaneous.
What is a non-spontaneous reaction?
You are conducting an experiment that requires salt water. You dissolve 0.435 mol of NaCl in 200mL of water. What is the concentration?
What is 2.18mol/L?
If 5.00 mol/L NaClO3(aq) is used to make 50.0 mL of a 3.75 mol/L solution, find the volume of the original solution.
What is 37.5mL?
Balance the combustion reaction for 1-butene. How many moles of CO2 are produced per mole of 1-butene?
C4H8 + 6O2 --> 4CO2 + 4H2O
Name this structure:

What is 4-ethyl-3-methyl-2-hexene?
Label the reactions occurring at each electrode in this plating apparatus. (Pt is Platinum and it forms a 2+ ion)
Pt electrode: Pt(s) --> Pt2+(aq) + 2e-
Iron key: Pt2+(aq) + 2e- --> Pt(s)
This is the number of protons, neutrons, and electrons in Li+
What are 3 protons, 4 neutrons, and 2 electrons?
How many liters of 4 M solution can be made using 100 grams of lithium bromide?
What is 0.3L.
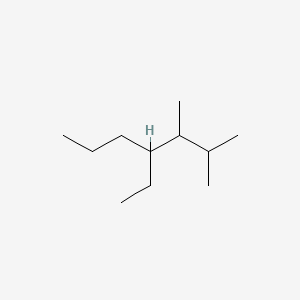
Name this structure:
What is 3-ethyl-4,5,6-trimethyloctane.
Name this structure:

What is 2,2,6-trimethylheptyne?
Identify the reduction and oxidation half-reactions in the following cell:

Reduction: Ag+(aq) + e- --> Ag(s)
Oxidation: Mg(s) + 2e- --> Mg2+(aq)
Draw the Bohr and Lewis diagrams for Mg.