Alkanes
Functional groups
Classifications
Naming
Drawing
What's Wrong
100
C2H6
Ethane
100
-OH
Hydroxyl
100
-C=C-
Alkene
100

Propene
100
Ethyne
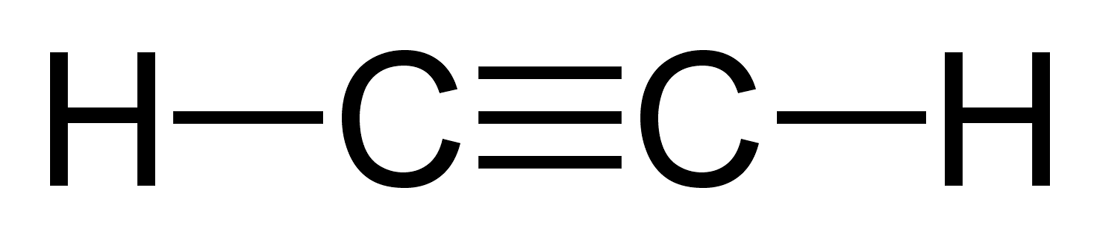
100
Methene
Ony 1 carbon, so cannot have a double bond
200
C4H10
Butane
200
-COOH
Carboxyl
200
R-X
(X= F, Cl, Br, or I)
Halogenoalkane
200
Butan-2-ol
200
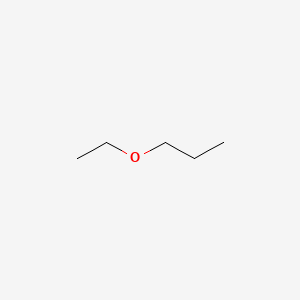
Ethoxypropane
200
Butan-1-al
Aldehyde has to go on the end, so the 1 isn't needed
300
Octane
C8H18
300
Carbonyl
R-CO-R'
300
Ketone
R-CO-R'
300
Propanoic Acid
300
Pentanal
300
Ethan-2-ol
No matter which carbon the -OH is on, it is the first one. Since there is only one -OH, the number is not needed.
400
C3H8
Propane
400
-CO-OR
Ester
400
R-COOH
Carboxylic Acid
400
2-methylbut-2-ene
400
Ethan-1,2-diol
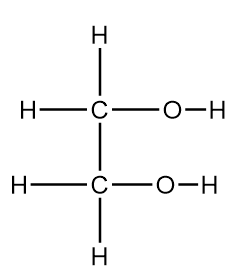
400
2,2-methylpropane
The 2,2 indicates that there should be 2 methyl groups, so it should also have the prefix "di"
500
What is the general formula for the Alkane homologous series?
CnH(2n+2)
500
Phenyl
-C6H5
500
Secondary Alcohol
500
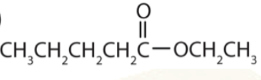
Ethyl Pentanoate
500
1,3-dichloropropene

500
2-ethylpropan-2-ol
The "ethyl" group attached to the 2nd carbon makes the new longest carbon chain 4, so it is no longer a base of propane