How far has a student travelled if their speed changed from 10m/s to 20m/s at an acceleration of 3 m/s2? (include units)
x = (v^2-u^2)/(2*a)
50 m
List 5 different energy stores
Nuclear, Tension, thermal, chemical, gravitational, kinetic
An isotope is a variety of an element with a different number of, what?
What is neutrons?
An ion does not have the same amount of _______ and ________.
What is protons & electrons?
Is the moon a luminous or non-luminous object?
non-luminous
If a student is walking around in a circle at a constant pace, what happens to their speed and velocity?
Their speed stays the same while their velocity constantly changes.
State the energy stores and transfers involved in turning on an electric fan.
chemical > electric > kinetic (Also thermal and sound)
The name of an isotope is the name of the element followed by a dash (-) and _____ __________ of the isotope.
What is atomic mass of the isotope?
An ion is an atom with a______?
What is a charge?
What is the unit we use to measure the pitch of sound?
Hertz
Which of the following graphs represents a car travelling on a motorway with cruise control on.

top middle or bottom right
Use the Sankey diagram to describe why the CFL has a higher energy rating. Be specific.
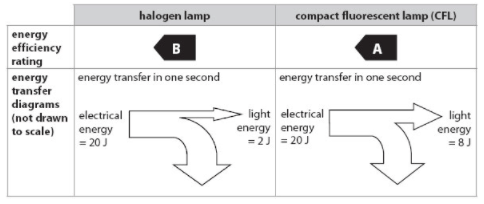
4 times as much usable energy.
Stable isotopes have a “happy” balance of ______ and _______.
What is protons & neutrons?
The name of radiation that can kick out electrons from orbits.
Ionising radiation
What is produced by anaerobic respiration?
Lactic acid
An ice skater pushes harder with his leg muscle, then he begins to move faster. Which law? and why?
2nd, because an increase in force increases acceleration
Explain why an insulated thermos can both keep cold drinks cold and hot drinks hot.
Creates a system which minimizes heat transfer (either way)
An isotope releases energy in order to become stable. This process is called ___ ______.
What is radioactive decay?
When an atom gains an electron, it gains a ____charge
What is negative?
Does a sound wave travel fastest in a solid, liquid, or gas?
Solid
What is meant by the term 'inelastic collision' in terms of energy and momentum?
KE is lost, momentum is conserved.
Calculate the height of a ball if it is has a velocity of 10 m/s just before impact.
G= 10m/s2
KE=1/2mv2
GPE=mgh
5 m
isotopic notation of an argon atom that has 22 neutrons
What is 40 Ar
How does an atom release light waves?
Electrons are excited to higher shells. When they return to their normal orbit, they release EM waves.
Why does hot air rise above normal temperature air?
Heat causes expansion and the air becomes less dense.