How many significant figures are in 0.004560?
4
How many H atoms in dihydrogen phosphate?
di = 2
What unit is pressure most commonly measured in for gas law problems?

What molecular geometry is shown?
linear
What is the molar mass of water? (Include units)
18.02g/mol
Which of these is hydroxide:
a. OH-
b. CN-
c. H2O
d. H2
a
What are the values at Standard Temperature and Pressure (STP)?
What is the electron geometry of a molecule with 3 electron domains?

trigonal planar
How many atoms are in one formula unit of Al₂(SO₄)₃?
17
Write the chemical formula of dinitrogen trioxide
N2O5
What is 25 degrees celsius in Kelvin?
298.15 K
Draw a Lewis Structure for water.
What is Avogadro's Number?
6.022 x 1023
Name any of the diatomic molecules.
Br2 I2 N2 Cl2 H2 O2 F2
What happens to pressure when volume decreases at constant temperature?
Pressure increases
A molecule has 2 bonds and 2 lone pairs. What is the electron geometry?
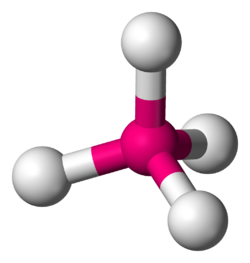 tetrahedral
tetrahedral
How many moles are in 18.0 g of glucose (C₆H₁₂O₆)
0.100 mol
What charge do phosphate and phosphite have?
3-
What is the Ideal Gas Law?
PV = nRT
Draw a Lewis Structure for CCl4
