1 mol @ STP is how many L?
22.4 L
System gains thermal energy while the work is done by the system. Indicate if Q and W are either endo thermic or exothermic.
Q= endo
W= Exo
Which of the 4 following sets of quantum numbers is wrong?
- n=4, l=3, ml=2
- n=3, l=1, ml =-1
- n=1, l=0, ml=-3
- n=3, l=3, ml=2
3
Give the correct electron configuration for the following elements/ions. How many valence electrons?
Zr
Cd
As
[Kr]5s24d2
[Kr]5s24d10
[Ar]5s23d104p3
Give Bond angle of molecule with Tetrahedral Shape
109. 5 ish
Combined Gas Law vs Ideal Gas Law? (equation)
P1V1/T1= P2V2/T2
Pv=nRT
A reaction has 543 J of energy done onto it while releasing 1211 J of heat. What is the ΔE of the reaction?
-668 J
Calculate the frequency of a wave of light with a wavelength of 589 nm.
5.09 x 1014 s-1
Put the following atoms/ions in decreasing order of atomic size:
K+ S-2 Ca2+ Cl-
S-2 > Cl- > K+ >Ca2+
Determine Molecular geometry and Electron Geometry of
ClF3
EG: Trigonal Bipyramidal
MG: T-Shaped
What is the molar mass of a 21.4 g of a gas in a volume of 18.48L at 125oC and 2.1 atm?
18.01 g/mol
A reaction does 675 kJ of energy onto its surroundings while releasing 1543 J of heat. What is the ΔE of the reaction in J?
-676543 J
Calculate the wavelength in nm of a wave of light that has a frequency of 3.22 x 1012 s-1.
9.32 x 104 nm
Put the following elements in order of decreasing metallic character
Ir C Ge Ba
Br > Ir > Ge > Ba
Draw a shape, and indicate Molecular Geometry, Electron Geometry, and Hybridization of:
ICl5
---- I have the picture on my sheet lol ---
What is the density of 0.391 g of SO2 at 750 mmHg and 22oC?
2.61 g/L
A pot of 2.34 L of water is heat from 25oC to 90oC. How much heat did the water absorb? The specific heat capacity of water is 4.18 J/goC, and the density is 1.00 g/mL.
636 kJ
A laser has a wavelength of 432 nm contains 4.21 mJ of energy. How many photons does it contain?
9.15 x 105 photons
Lewis structure for
SO42-
----Picture----
Draw a shape, and indicate Molecular Geometry, Electron Geometry, and Hybridization of:
ClO3-
---- I have the picture on my sheet lol ---
A gas mixture contains 0.70 mol of N2, 0.20 mol of H2, and 0.10 mol of CH4. (1.) What is the mole fraction of H2 in the mixture? (2.) Calculate the pressure of the gas mixture and (3.) the partial pressure of each constituent gas if the mixture is in a 10.0 L vessel at 27oC.
nH2= 0.2, nN2= 0.7, nCH4= 0.1
1. XH2= 0.2 mol
2. Ptot= 2.46 atm
3. xH2= 0.492, xN2= 1.722, xCH4= 0.246
A piston is expanded from a volume of 0.221 L to 1.06 L with an external pressure of 1.24 atm. How much work is done in Joules? (1 L*atm = 101.3 J)
-105 J
Calculate the wavelength of an electron traveling with a speed of 3.22 x 107 m/s in nm
2.26 x10-2 nm
Lewis structure for Cl2CO
---Picture---
Hybridization and Bond angle C:1,3,5,6
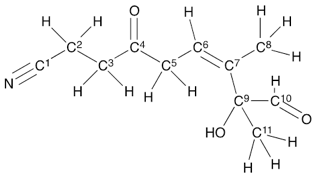
1: sp, 180
3: sp3, 109.5
5: sp3, 109.5
6:sp2, 120