Water (not snow or steam) is an example of what state of matter?
Liquid
How many types of particles are there?
a) 1, Particles duh
b) 3, Atoms, Molecules, and Subatomic particles
c) about 6 or 7
C) about 6 or 7
just kidding
b) 3, Atoms, Molecules, and Subatomic particles
True or False: This picture is NOT of an atom.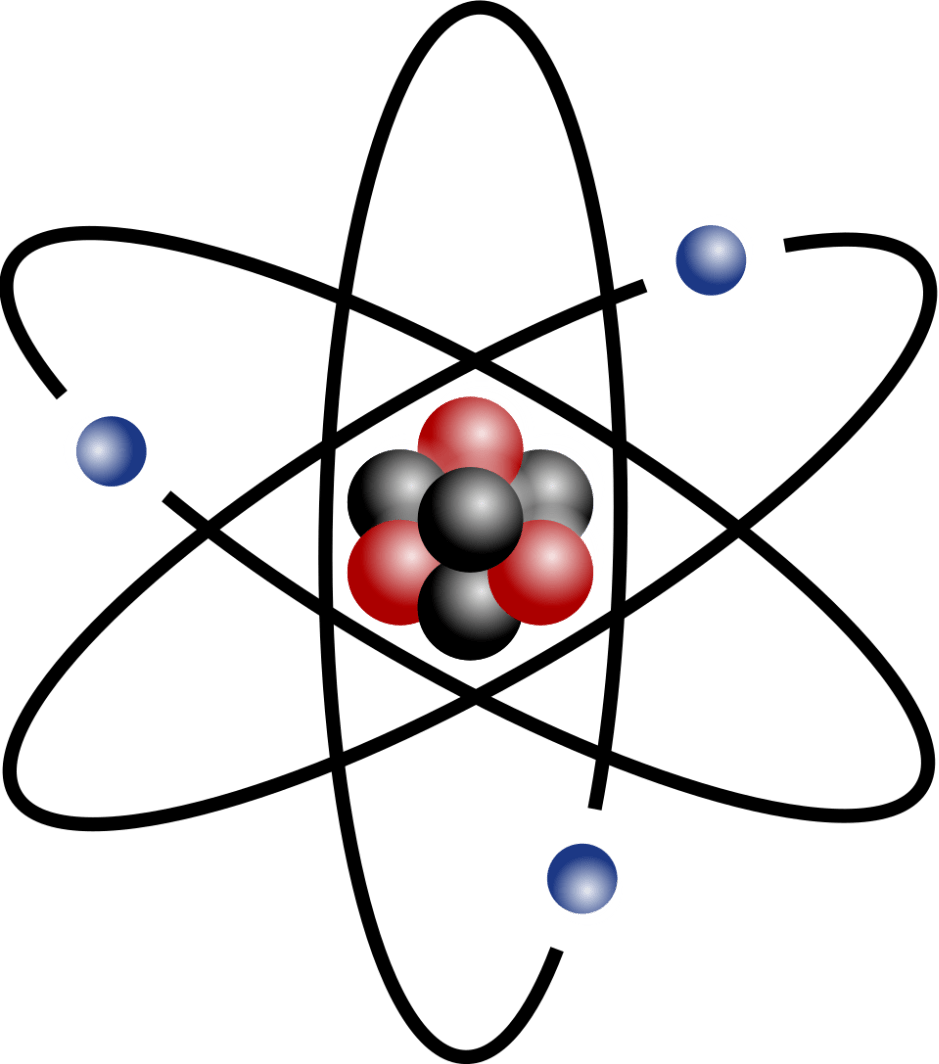
False. This picture is an atom.
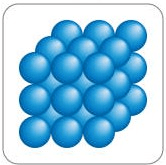 This is an example of the particles for What state of matter?
This is an example of the particles for What state of matter?
Solid
false. Particles are smaller than bacteria and other microscopic objects/organisms.
The centre of an atomic particle (where protons and neutrons live) is called the?
Nucleus!
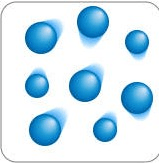
Gases
True or false: Protons are identified as a circle with a + symbol inside
True!
True!
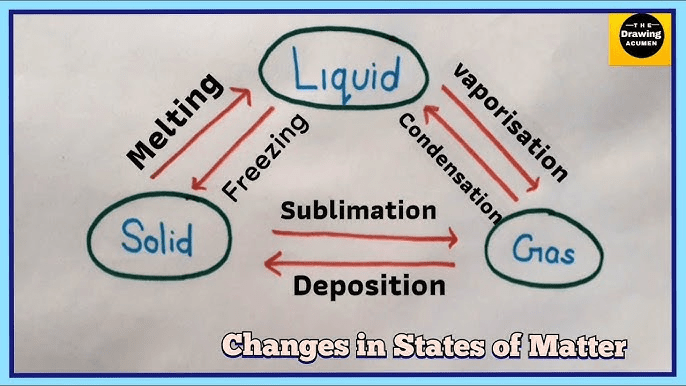
True or False: Changes in states of matter.
Solids cannot change states into a gas without becoming a liquid first.
This particle; H20, is an examples of what kind of particle:
a) Molecule
b) Atom
c) Proton
a) molecule
What subatomic particle in atoms has no special symbol to identify it?
Neutrons!
a) Have particles that are close together
b) Match the shape of it's containerC) Gasses expand and fill their container
There are how many different types of Atomic particles
a) 3
b) 95
c) 118
c) 118, one for each element on the periodic table
What part of an atom do you think weighs the most?
The Nucleus does! Protons and neutrons are (relatively) heavy!