What do elements in the same group share?
Similar Chemical and Physical Properties
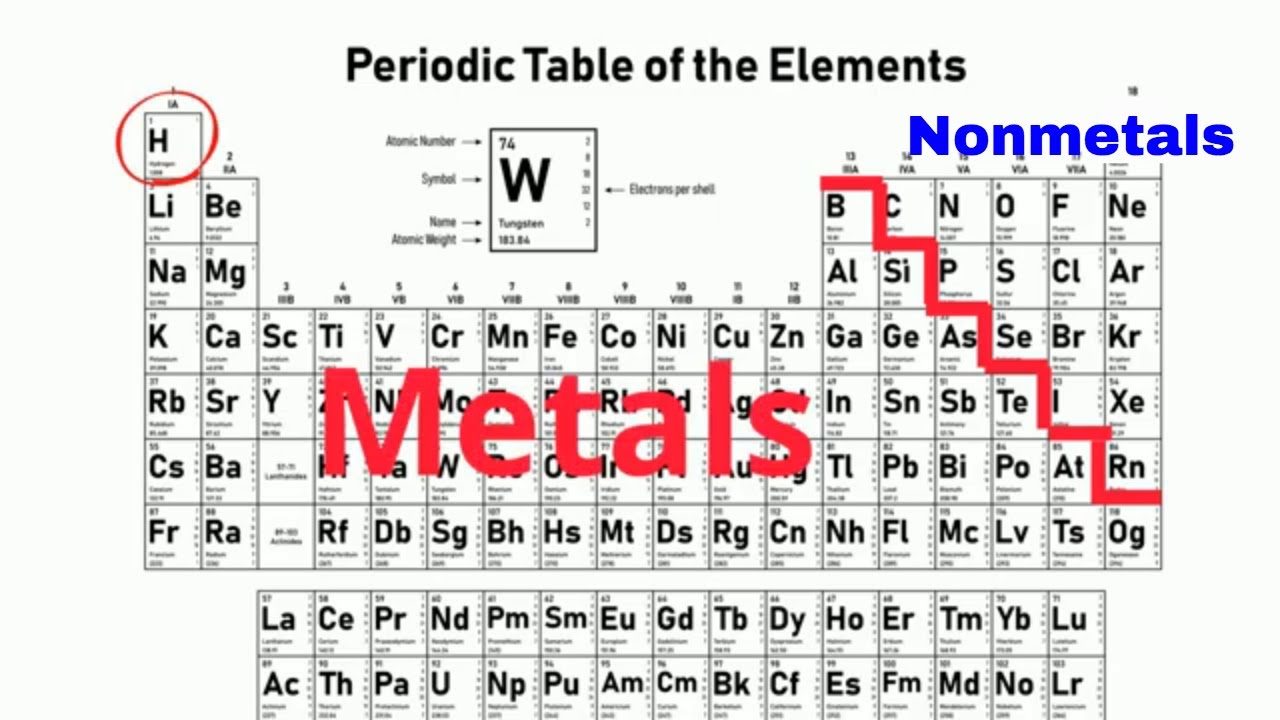
What separates the metals and non-metals?
The bolded, zig-zag line
What is a row going left and right called on the Periodic Table?
A period
Sulfur has an atomic number of 16. What does the atomic number of 16 tell you about Sulfur?
The number of protons Sulfur has in its nucleus
How are the elements arranged on the Periodic Table?
Which family do Calcium and Barium belong to?
Alkaline Earth Metals
Draw the zig zag line. Label the correct sides with Metals, Non-metals and Metalloids.
Which family is group 17?
Halogens
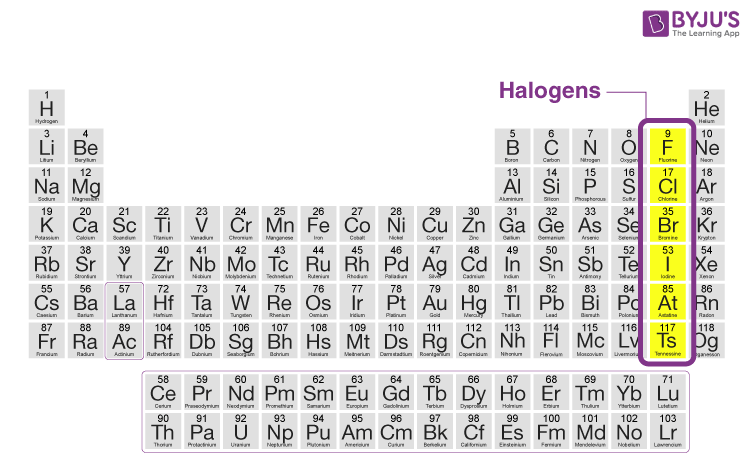
Find Bromine on the Periodic Table. What is the atomic number of Bromine?
35

The ease and speed at which an element combines with other elements describes the element's _________________?
Reactivity
What are the 3 reactive families? Circle the most reactive family
Alkali Metals (most reactive)
Alkaline Earth Metals
Halogens
Out of these options: Selenium (Se), Iodine (I), Zinc (Zn) or Argon (Ar)
Which would be the best conductor of electricity?
Zinc because it is a metal!
Where are the metalloids located?
Bordering the zig-zag line
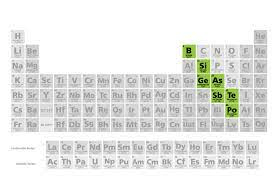
How many valence electrons would Tin have?

Group 14 = 4 Valence Electrons
What is it called when a metal can be hammered or pressed permanently out of shape without breaking?
Malleability
Which family would provide the best materials for making jewelry? HINT: Jewelry is able to be hammered, bent, molded and is typically unreactive.
Transition Metals because the Alkali and Alkaline Earth Metals are Reactive, Rare Earth metals are unstable, and Halogens and Nobel gases are non-metals.
What is it called when a metal can be pulled into a wire?
Ductile
What is the 3rd element in the Alkali Metals?
Potassium

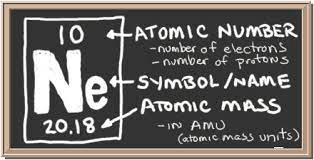
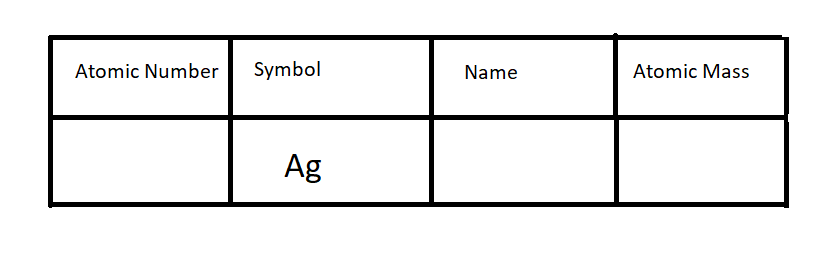
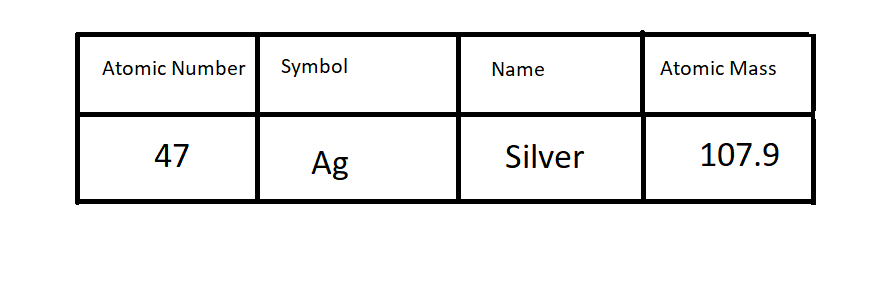
Write this element and label each section of the atomic square with the following: Symbol, Name, Atomic Mass and Atomic Number.

Who was the first scientist accredited with developing the Periodic Table of Elements?
Dmitri Mendeleev
What do the following elements have in common?
Lithium (Li), Sodium (Na), Potassium (K), Rubidium (Rb), Cesium (Cs) and Francium (Fr)
*Similar physical and chemical properties
or
*Same number of valence electrons
You get a dull, yellow solid that breaks into powdery pieces when hit with a hammer. How would you classify this sample? (Hint: think of the category name?)
Non-Metal because it has opposite properties to metals
Which element is in group 15 period 4?
Arsenic

Fill out the rest of the data table
What determines how reactive an element is?
The number of Valence electrons
