Nonmetals
This states that matter cannot be created nor destroyed only transformed.
What is Law of Conservation of Mass/Matter?
This is found on the left side of the yields sign. In other words, what goes into the reaction.
What is a reactant?
How many electrons will the Strontium atom give away to become a cation?
2
This group of elements tends to give away one electron to become a stable ion.
Group 1
I tend have very high melting and boiling points.
Ionic compounds
2H2 + 2O2 >>> 2H2O
Do I honor the law of conservation of matter/mass?
No, you don't.
Does this is image support the law of conservation of mass?
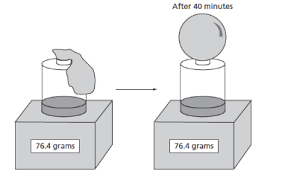
What is yes?
What's the chemical formula of Beryllium Sulfide?
For the main group elements, the number of valence electrons can be determined by:
the ones place of the group number
I am formed when electrons are transferred between atoms, resulting in a neutral compound due to the balancing of positive and negative charges.
What is an Ionic compound?
The masses of the reactants and the products in a chemical reaction according to the Law of conservation of mass should always be ____.
What is equal?
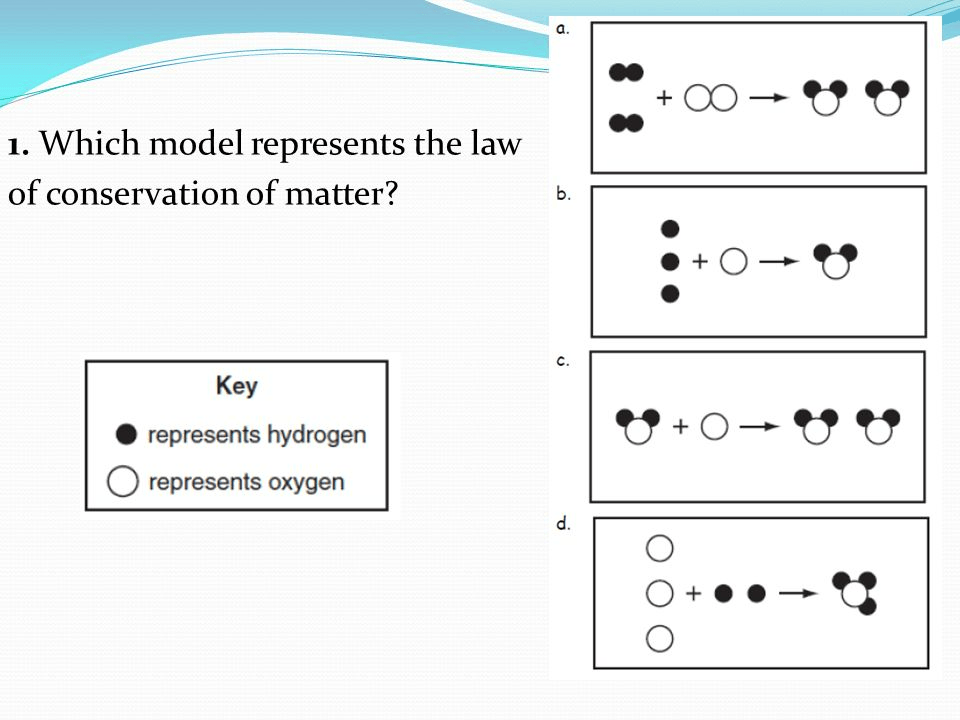
What is A?
A compound has a melting point that is over 500-degree Fahrenheit. When it in an aqueous solution is conducts electricity. It is a green dusty powder that has a fruity scent. It is most likely a(n) ionic or covalent compound?
It is ionic.
This group of elements tend become cation with a 3+ charge.
What is group 13?
Although you call me water (H2O), in science, my name is
Dihydrogen Monoxide
12 grams of reactant X react with 15 grams of reactant Y. This is the total mass of the product(s).
What is twenty seven (27) grams?
Double Jeopardy Question!
Does this image support the law of conservation of matter/mass? what is the total mass of the product and reactant?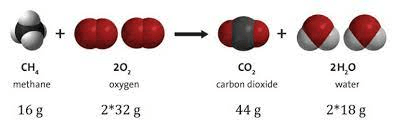
Yes. Reactants= 80g Products= 80g
I'm soap.
The chemical formula C17H35COO .
How many atoms make up one molecule of me
What is 55?
Double Jeopardy Question
When sodium reacts with an element from this group, it tends to form a stable binary compound that fits this template: Na2X
where X could be any element from the group.
What is Group 16?
Yes! that's my chemical formula! I'm aluminum oxide.
What is Al2O3 ?
In a chemical reaction, 50g of Chlorine is used to produce 95g of Sodium Chloride. How much Sodium was used?
What is forty-five (45) grams?
Does this image support the law of conservation of matter?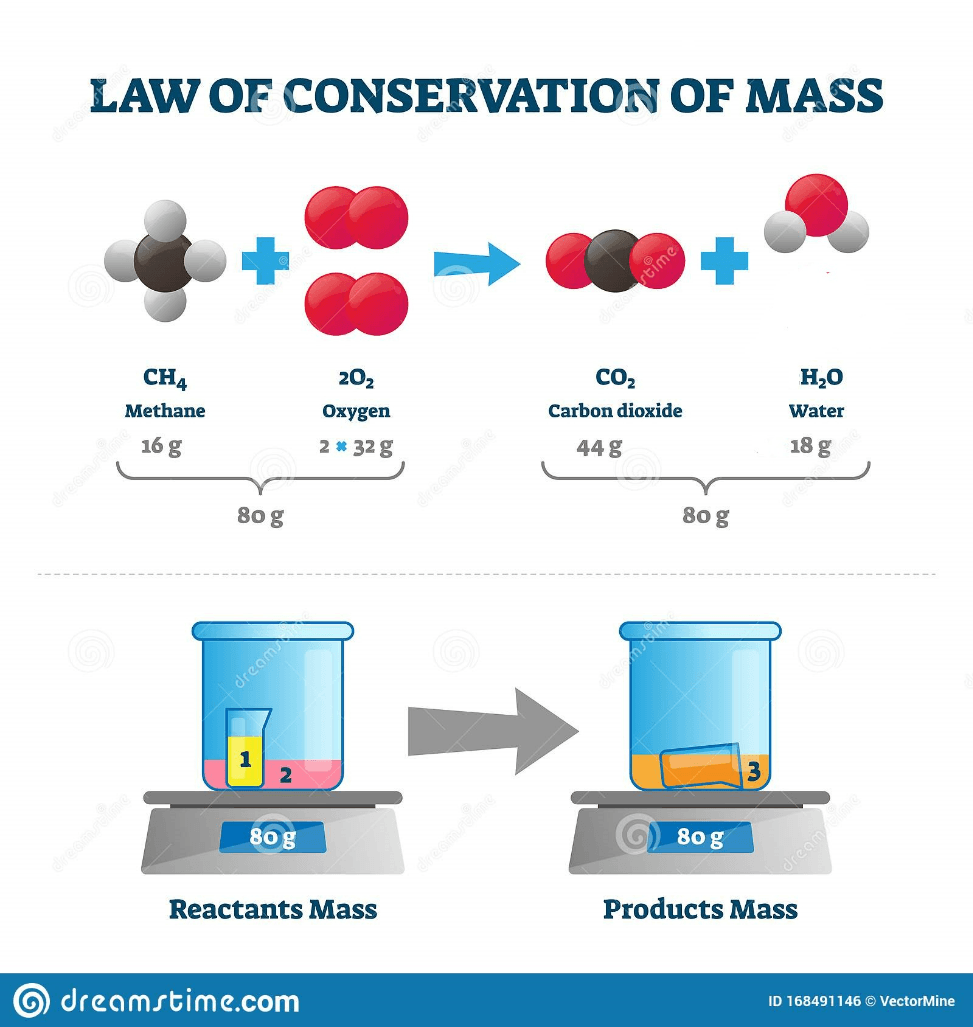
No, it does not -- the red atoms are not balanced!
Like Beyonce said -- say my name, say my name!
N2O4
What is dinitrogen tetroxide?