Atoms go in order on the periodic table by their....
Atomic # (or number of Protons)
List two properties of Chromium (atomic # 24).
Two of the following are correct: shiny, highly conductive, malleable, highly reactive
All atoms in the same period have the same number of....
energy levels
To draw a Bohr model do you look at the Period # of the Group #?
To draw a Lewis Dot structure for an atom do you look at the Period # or the Group #?
Group #
Metals can be found on the ____ side of the periodic table.
Left side
Why can't we predict the properties of Boron?
Because it is a metalloid (they have properties of both metals and nonmetals)
All atoms in the same column have the same number of....
valence electrons
What are energy levels?
The "rings/shells/orbits" around the nucleus of an atom where electrons are found.
Draw the Lewis Dot Structure for Phosphorous
Make sure you have 2 e- on one side, and 3 single e- on the other sides!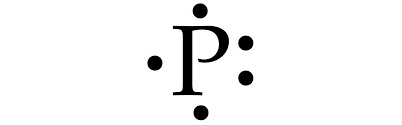
On the periodic table, mettaloids are found where?
In between metals and nonmetals (or along the "staircase".
List two properties of Phosphorus (atomic # 15).
Two of the following are correct: dull, poor conductor, brittle, not very reactive
What is the MOST reactive metal on the periodic table and the LEAST reactive?
Most = Francium
Least = Aluminum
What are valence electrons?
Electrons on the outermost energy level.
How many valence electrons do Group 17 elements have?
7 valence electrons
Noble Gases
Why does the size of the atom increase as you go down columns?
Because the number of energy levels increases.
Why does reactivity increase as you go down columns?
Atomic size increases as you go down columns, thus electrons are further and further away from the nucleus. This means the nucleus has a harder time "holding electrons in." Valence electrons can get "taken" by other elements very easily.
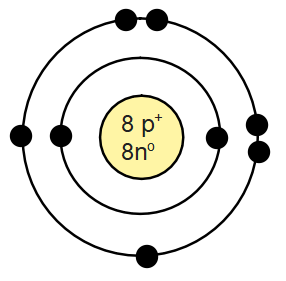
Draw the Bohr model of Oxygen, include the # of protons and neutrons in the nucleus.
Draw the Bohr model of Magnesium to prove why it is in Group 2.
Valence e- are circled. There are 2 valence e-, thus Magnesium is in Group 2.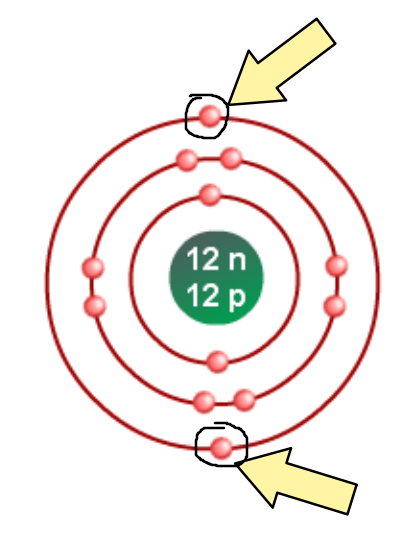
These types of metals make up the majority of the metals on the periodic table.
Transition Metals
All elements like to have a full outer shell with 8 valence electrons, except for this element.
Helium
Why does reactivity decrease as you go across rows?
As you go across a row the atomic # increases. This means the number of protons increases as well. With more protons in the center, the electrons are more attracted to the nucleus, and get "pulled in." This makes it harder for other atoms to "take" away its valence electrons.
How many electrons does it take to fill the second energy level of atoms?
8 electrons
What's the point of drawing Lewis Dot Structures?
They show the only e- that are involved in chemical reactions.