What element has an atomic mass of 69.723
What is Gallium
What elements are touching the "staircase" on the periodic table
Metalloids
Found in the nucleus of an atom.
Protons + neutrons
How many valence electrons does carbon have?
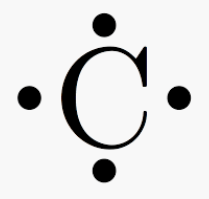
4 -Four
Good for you!
Just kidding. You get 100pts.
This number stands for the number of protons.
What is the atomic number
The majority of the periodic table is...
metals
Neutrons and protons make up the nucleus and equal _.
What is the atomic mass?
Oxygen has _ (number) valance electrons.
What is 6 (six)?
How many periods are on the periodic table?
7
The columns of the periodic table are known as?
What is the group
This term means that the element can be bent, shaped, or flattened into a sheet
malleability
Determines the element's identity (atomic number)
DOUBLE POINTS
What are protons?
The number of valence electrons determines this about an element.
How many valence electrons does Krypton have?
8
What element is found on Group 4 period 5?
Ziconium
What family does Chlorine belong to?
Halogens
The only element with no neutrons
Hydrogen
Elements in Group 1 have this many valence electrons.
What is 1?
Chlorine, Bromine, and Iodine have the same chemical properties because they have the same
valence electrons
These elements do not conduct heat or electricity.
What are non-metals?
Uh oh...
You automatically gain 500 points.
Which is not a non-metal from the following: Boron, Iron, Hydrogen, Argon?
Iron
These elements have a full (complete) valence shell.
What are Noble Gasses (Group 18)?
Group number and period number that Copper (Cu) is in.
What is group 11, period 4?