How many ELECTRONS can the first ring/orbital shell hold?
How many ELECTRONS can the second ring/orbital shell hold?
The first ring/orbital shell can hold a maximum of 2 ELECTRONS
The second ring/orbital shell can hold a maximum of 8 ELECTRONS
How does the radii move on the Periodic Table - left to right?
largest to smallest
On a periodic table, what do rows represent
Each row indicates how many rings/shells an atom has.
An atom’s mass number is 210 and its atomic number is 85. How many neutrons does the atom have?
125
ion
What is the number of Neutrons located in this element? Round if necessary.

12 neutrons.
What is mass number
Number of protons and neutrons
How is the table organized?
What are 3 physical properties of metals?
luster, malleable, ductile, good conductors, donate electrons, high density, high melting point
The first person to call atoms "atomos" was
Democritus
Draw the Bohr model of magnesium
check on board
What is the name of the 4 main classifications on the periodic table?
metals, non-metals, metalloids and synthetics
What two elements are liquids at room temperature?
Mercury and Bromine
Why is the atomic mass not a whole number?

What are three physical properties of non-metals?
not shiny, not malleable (brittle), not ductile, poor conductors (good insulators), gain electrons, low density, low melting point
What category does the image represent?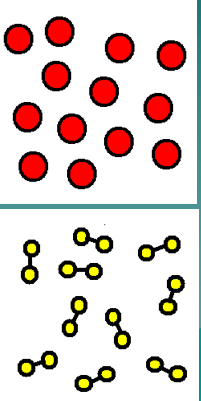
An Element and a Compound
Draw the Lewis dot structure for silicon
on board
What is smallest sub-atomic particle?
Electrons
What has the symbol Ne, Na, K?
Neon
Sodium
Potassium
What is a Mixture?
Two or more substances that are mixed together but are not chemically bonded.
An element that has differing numbers of neutrons is known as
isotope
What is the name of the group 7A, 17 family?
halogens
How many elements have been named?
118
Which subatomic particle never changes?
protons
Which family (name not number) is the most reactive metals family?
Alkali Metals
What is the least reactive family on the Periodic Table? Also, name what column number.
Noble Gases, Col. 18
Based on the placement on the Periodic Table, what element has two valence electrons and two energy levels?
beryllium
What is the charge of the nucleus? Why... how do you know?
Positive