These rows have the same number of energy levels or shells and chemical properties are NOT all similar.
What are periods?
These elements have properties of both metals and nonmetals.
What are Metalloids?
This is the basic unit of chemical element
Atoms
These columns have the same valence electrons and chemical properties are VERY similar.
What are Groups or Families?
These group is the most reactive while this group is the most inert (stable)
What is group 1 and group 18?
These elements have no luster (dull), are brittle (breaks easily), and are a poor conductor of heat.
What are nonmentals?
These elements are solid at room temperature (except mercury), have a shiny luster, are ductile(bend into a wire), and malleable.
What are metals?
These elements are good conductors (heat and electricity), have high density, high melting points, and have more reactivity (the elements at the bottom left corner of the periodic table are the most reactive)
What are metals?
These elements are not ductile, not malleable, have low density, a low melting point, and are less reactive than metals.
What are nonmentals?
What is reactivity?
Sodium (Na) is a highly reactive element. Which characteristic of sodium is responsible for its reactivity?
It has one valence electron.
This is the outermost shell of an atom.
What is a valence electrons?
Of the four atoms below, which two would be in the same Group?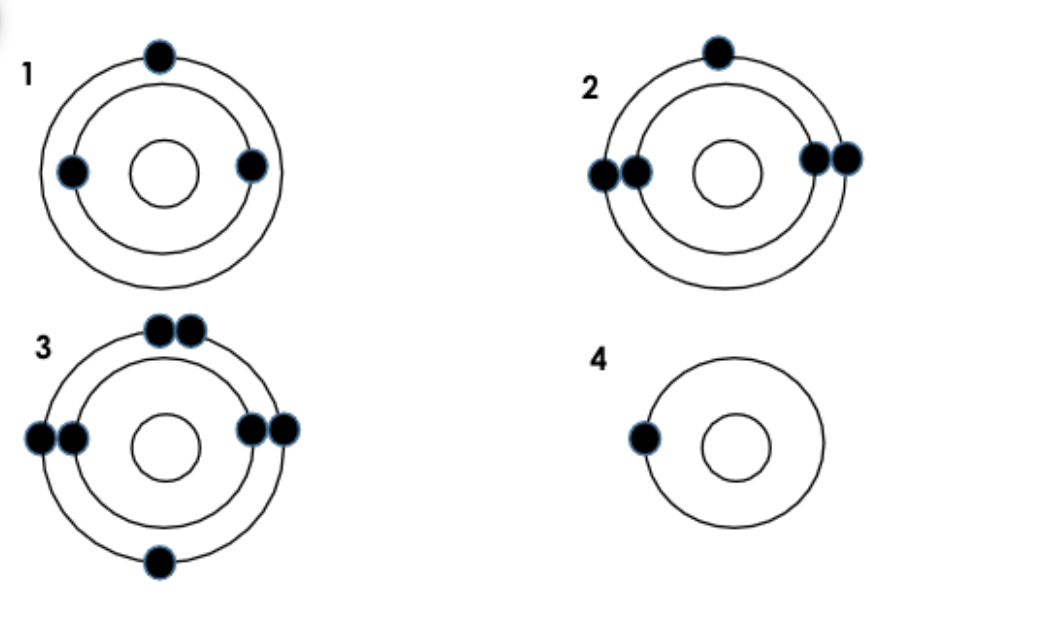
1 & 4
This is what participates in forming chemical bonds with other atoms.
What is valence electrons?
The atomic mass is the sum of these two things.
What are protons and neutrons
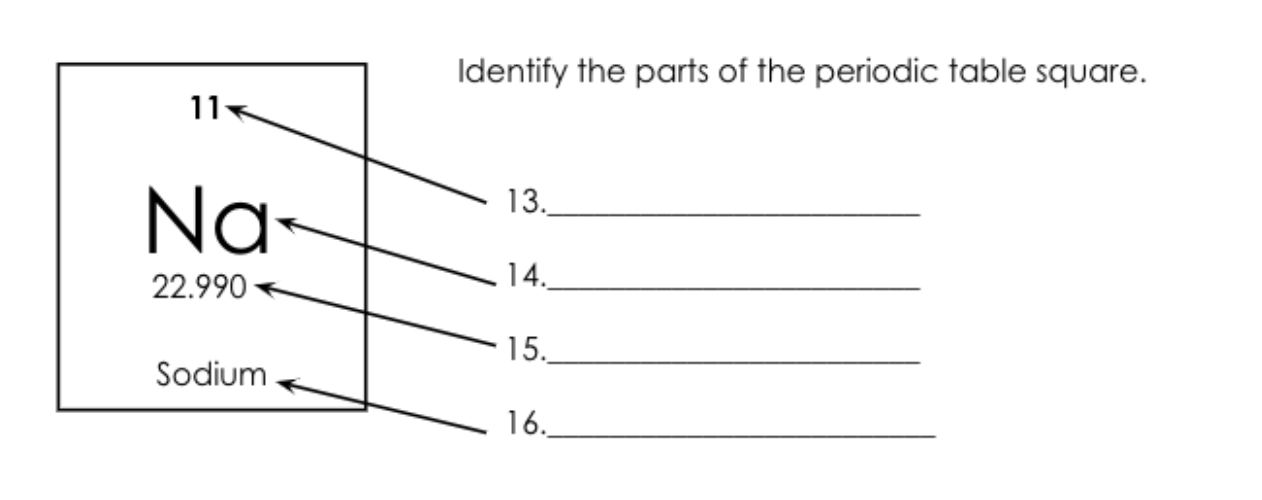
What is number 14 called?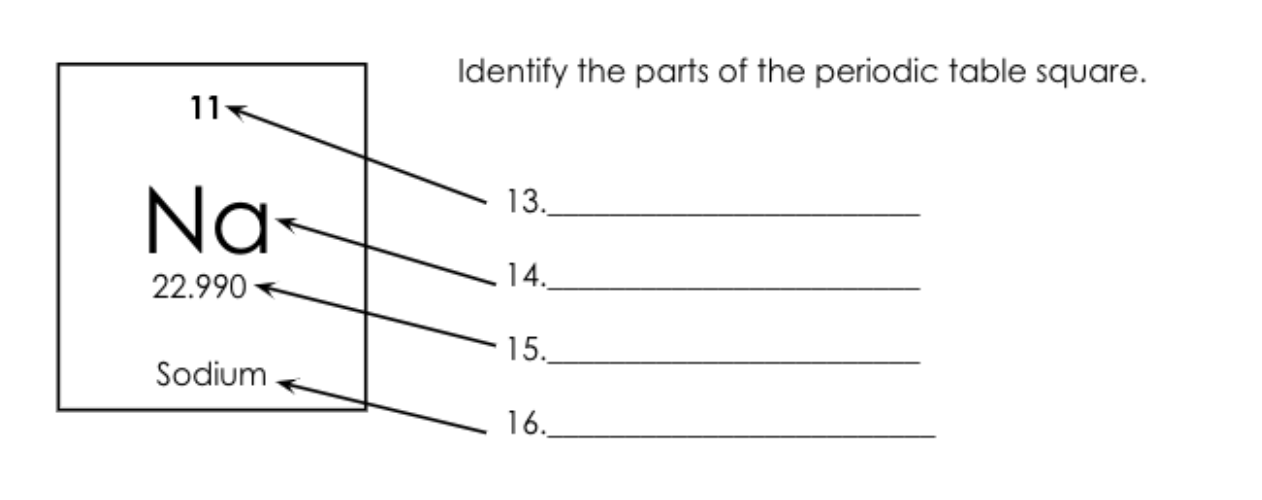
Atomic Symbol.
What is number 15 called?
Atomic Mass = Protons + Neutrons
Which two elements have one valance electron?
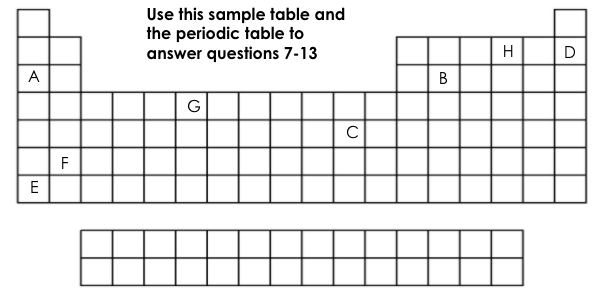
A&E
What is number 13 called?
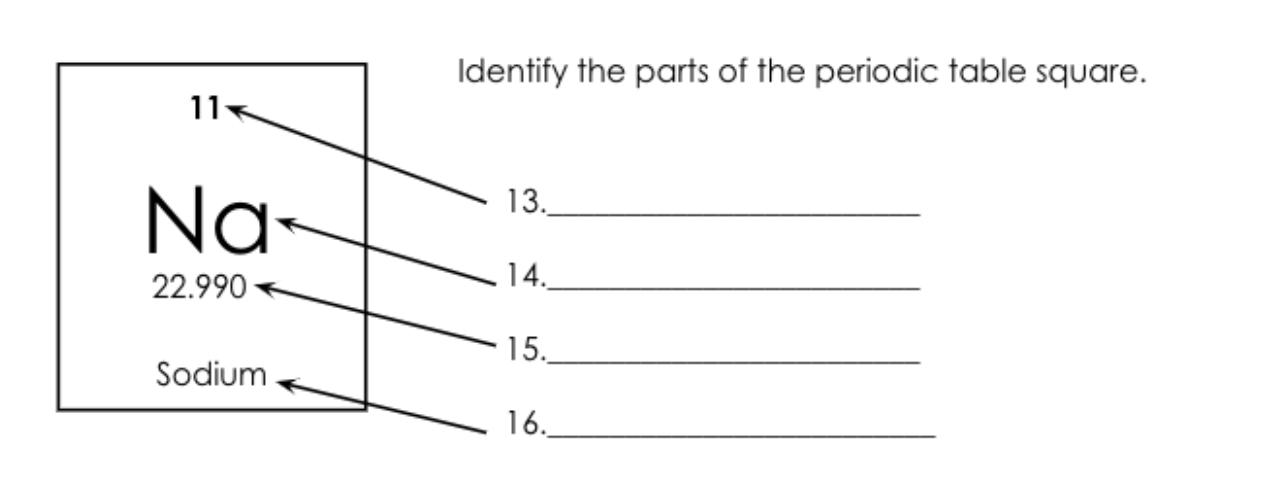
Atomic Number = Protons/electrons
What is number 16 called?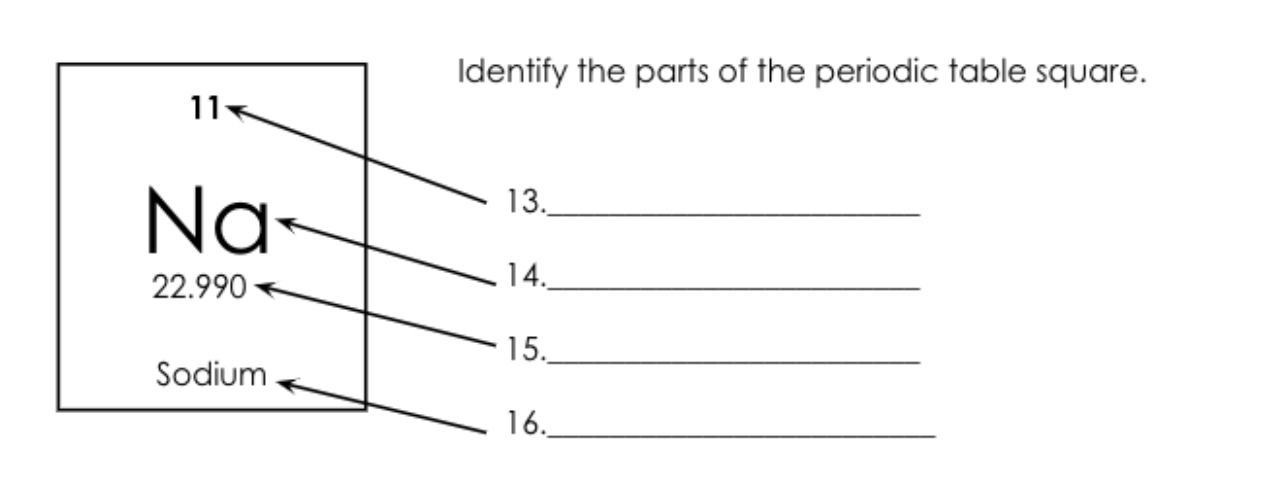
Element Name
Which element is chemically stable (inert)?
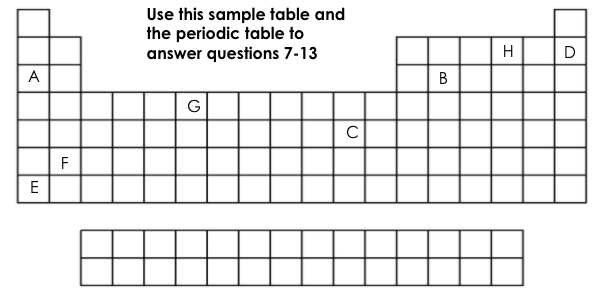
D
Which element is a metalloid?
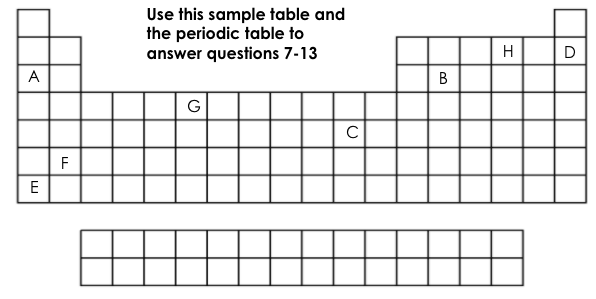
B
Which element has 6 valance electrons?
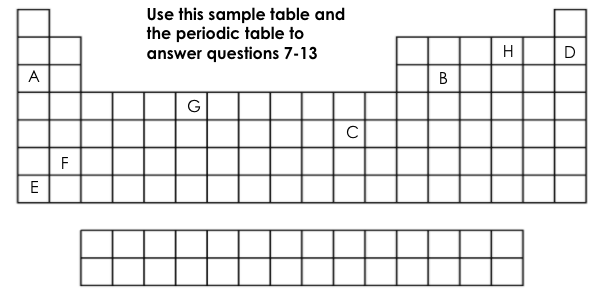
H
What two elements have the same number of energy levels (shells)?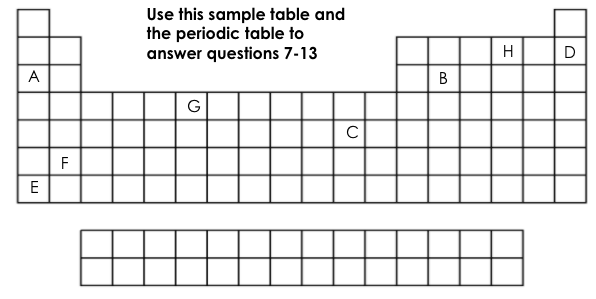
D&H or A&B
Which element is the most reactive on this table?
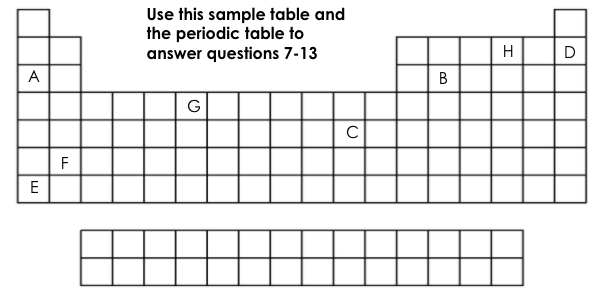
E