This group lacks properties of metals.
What are non-metals?
The number of protons gives this number, which is unique to each element.
What is the Atomic Number?
What is a compound?
Elements with similar properties are organized into these vertical columns.
What are groups?
In a Lewis dot diagram, these are represented as dots.
What are valence electrons.
This element in in Group 1 and Period 1.
What is Hydrogen?
This group fills in groups 3 through 12.
What are transition metals?
These particles are part of what gives an atom its mass, but have no charge.
What are Neutrons?
This kind of chemical bond is between a metal and a non-metal, where electrons are transferred.
What is an ionic bond?
Elements with the same valence energy level share the same one horizontal row, called this.
What is a period?
In a Bohr Model of an atom, electrons are drawn on these circles, which represent these.
What are energy levels?
Nitrogen is in this Group Number.
What is Group 15?
This group has 1 valence electron.
What are Alkali Metals?
Phosphorus has this many neutrons.
What is 16?
In this kind of chemical bond, valence electrons are shared between two non-metal atoms
What is a covalent bond?
What are non-metals?
The Bohr Model here represents this element.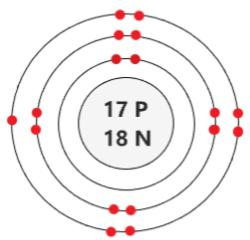
What is Chlorine?
Potassium is in this Period.
What is Period 4?
This group includes Calcium.
What are the Alkaline Earth Metals?
Atoms need to do this in order to have a -1 charge.
What is gain an electron?
The bond shown here is drawn with two lines, indicating it is one of these.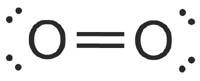
What is a double bond?
These elements form the "stair step" separating the left and right side of the Periodic Table.
What are the Metalloids?
According to this Lewis Dot Diagram, Sulfur needs this this many valence electrons to fill in its energy level.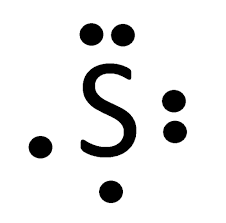
What are 2 electrons?
These two groups are part of periods 6 and 7, but are usually located below the rest of the table.
What are the Lanthanides and Actinides?
This group is the only one to contain solids, liquids, and gasses.
What are Halogens?
Atoms that have a positive or negative charge are called this.
What are ions?
This kind of bond allows electrons to flow freely between atoms.
What is a Metallic Bond?
He was the first to organize the elements into a table, sorting them by atomic weight.
Who is Dmitri Mendeleev?
These two elements have a full valence energy level with just 2 electrons.
What are Hydrogen and Helium?
This element is the only one that has properties of two groups.
What is Hydrogen?