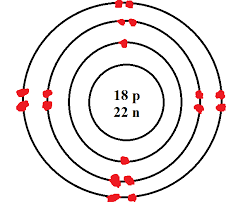
The elements in group 1 are known as __?
The elements in group 1 are known as __?
What is Oxygen
I have 4 rings with 2 electrons in my outer energy level.
Who is Calcium?
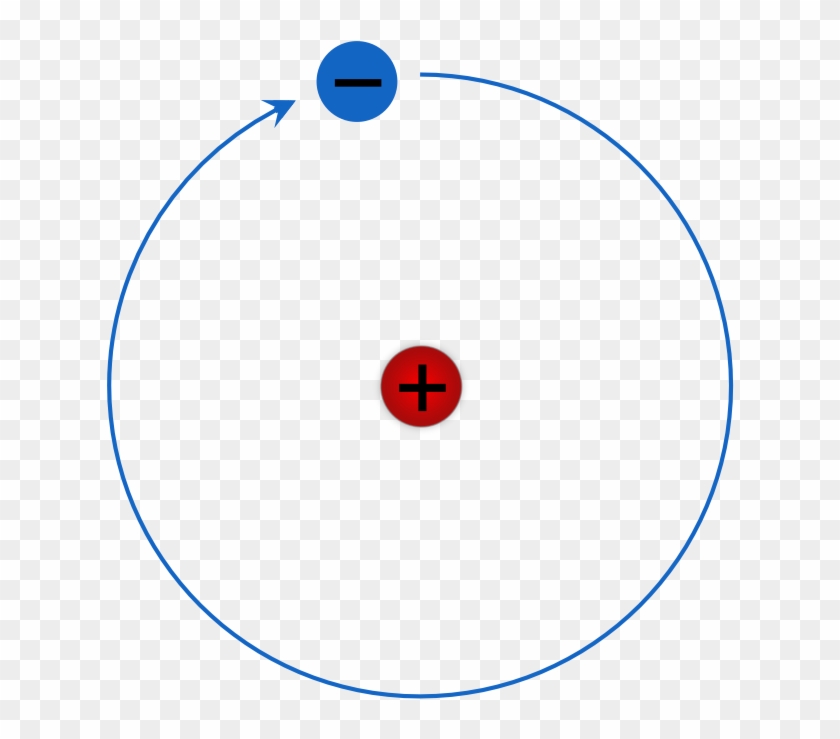
What is Hydrogen?
I have the smallest mass of the elements in the least reactive nonmetal group.
Who is Helium?
What is Argon?
This can tell you the number of rings or energy levels of an element?
What is a period?
What are groups?
What is Helium?
This is the formula for solving the number of neutrons
What is the atomic mass - atomic number
I am a very unstable, I am a gas, and I have 1 valence electron
Who is Hydrogen?
Something that helps electric currents or heat to flow more easily?
What is a Conductor?
The periodic table is arranged in order by what?
What is the Atomic number, or Protons

What is Potassium
What does the group number tell us?
What is the number of valence electrons
I have 4 rings and 8 Valence Electrons
Who is Krypton?
This has a negative electrical charge and is found outside the nucleus
What is an Electron?
What are the 3 groups of the periodic table
What are metals, nonmetals, and metaloids