Name the 3 subatomic particles and their charge
Proton - Positive
Neutron - No charge/Neutral
Electron - Negative
Which element has 1 valence electron?
Hydrogen
What period does Nitrogen belong to?
2
An atom’s mass number is 210 and its atomic number is 85. How many neutrons does the atom have?
125
What's the correct name for a compound made from lithium and fluorine?
Lithium Fluoride
What are 3 physical properties of metals?
luster, malleable, ductile, good conductors, donate electrons, high density, high melting point
The first person to call atoms "atomos" was
Democritus
On a periodic table, what are rows called?
periods
What name is given to the compound made of 2 elements?
Binary compound
Ex: Sodium Chloride
What is the chemical formula for Water?
H2O
What are three physical properties of non-metals?
not shiny, not malleable (brittle), not ductile, poor conductors (good insulators), gain electrons, low density, low melting point
What is smallest sub-atomic particle?
Electrons
What are valence electrons?
Electrons in the outermost shell of an atom
How is the table organized?
What is the number of Neutrons located in this element? Round if necessary.

12 neutrons.
What category does the image represent?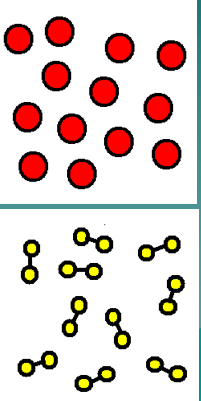
An Element and a Compound
How many ELECTRONS can the first ring/orbital shell hold?
How many ELECTRONS can the second ring/orbital shell hold?
The first ring/orbital shell can hold a maximum of 2 ELECTRONS
The second ring/orbital shell can hold a maximum of 8 ELECTRONS
The atomic mass measures the mass of the ________ and ________ in an atom
Number of protons and neutrons
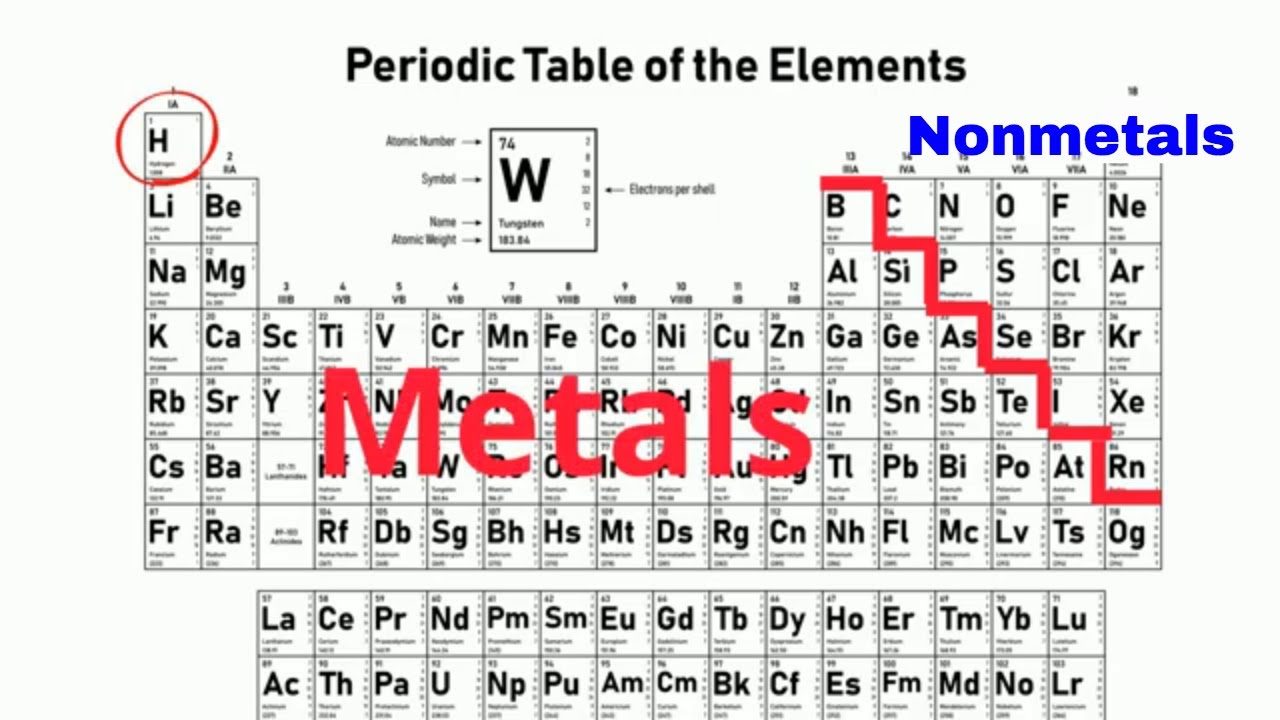
What is the name of the 3 main classifications on the periodic table?
metals, non-metals, and metalloids
What is the least reactive family on the Periodic Table? Also, name what column number.
Noble Gases, Col. 18
Name one element from the Halogen Family
fluorine, chlorine, bromine, iodine, astatine, and tennessine
What is the binary compound name for Calcium and Sulfur?
Calcium Sulfide
What is the family name of the group 17?
halogens
Which subatomic particle never changes?
protons
Name 2 diatomic molecules
hydrogen (H2), nitrogen (N2), fluorine (F2), chlorine (Cl2), iodine (I2), bromine (Br2) and oxygen (O2).
Which family (name, not number) is the most reactive metals family?
Alkali Metals
Name an element with 4 valence electrons
Carbon, Silicon, Germanium, Tin, and Lead
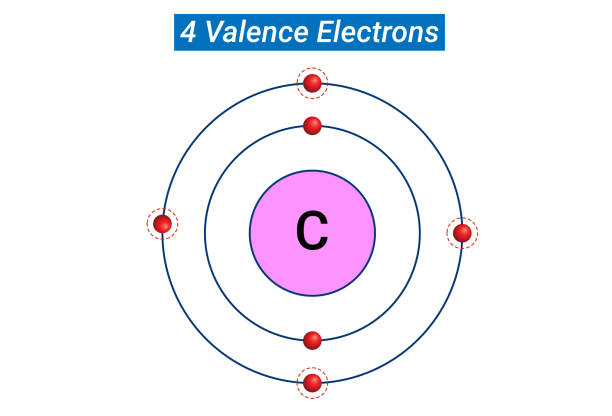
Based on the placement on the Periodic Table, what element has two valence electrons and two energy levels?
beryllium
Where on the periodic table can you find nonmetals?
on the right