Give 4 examples of chemical change.
rusting, burning, baking, tarnishing, digesting, rotting, decomposing, exploding (fireworks)...
Scrambling eggs, boiling water, dissolving sugar, dicing vegetables are 4 examples of what?
Physical Change
What is inside bubbles during a CHEMICAL reaction?
A NEW gas
In a __________ substances do not change into new substances.
physical change
In a chemical reaction, mass _______________
is conserved (stays the same)
During a chemical reaction, the atoms ________ to form ______.
rearrange; new molecules.
How many substances join to form new chemical properties?
Two or more
Give 3 evidences of physical change.
Change in form, change in shape, change in size, change in phase/state, dissolving, expected color change, expected temperature change
What occurs when two or more substances interact and the molecules change to from new substances?
A chemical reaction
Shredding paper is a ____________ because _______
physical change; it changes shape but is still paper.
This is a _______________ because _______ AND _______

Chemical reaction; two liquids formed a new solid; unexpected color change
In a chemical equation, the _____________ are the starting substances.
reactants
Salt water is a _______ change because _______
Physical change; it can be reversed (salt and water can be separated)...OR...the salt and water are evenly mixed. No new substances are added.
In a chemical reaction, the mass of the reactants is _____________ the mass of the products
equal to; the same as
When temperature INCREASES (gets warm) during a chemical reaction, thermal energy is ________
released
Mixing sand and water is
A physical change
In a chemical equation, the final substances are the ______________
products
When temperature DECREASES (gets cold) during a chemical reaction, thermal energy is ______
absorbed
Rusting is a chemical reaction. What EVIDENCE supports this claim?
Unexpected color change
Tie dye is a ________ change. What EVIDENCE supports this claim?
Physical; expected color change
During a chemical change ______ can be released in the form of heat, light, sound, electricity
energy
In a chemical reaction, molecules ___________
change into new molecules
This model represents a _____ change because ____________________
This could represent ____ + ____ = ______
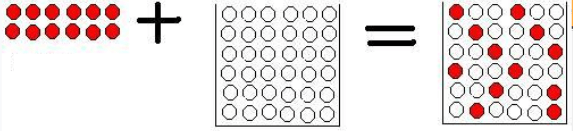
physical change; the substances are mixing but no new substances are formed; sugar + water = sugar water; salt + water = salt water, red food dye + water = light red water
Balanced or unbalanced? Why or why not?

unbalanced; not the same number of each type of atom on both sides.
2 blue reactant atoms, 3 blue product atoms; 4 red reactant atoms, 3 red product atoms; 2 gray reactant atoms, 1 gray product atom
The production of a solid when two liquids interact
precipitate
A symbolic representation of the reactants and products in a chemical reaction.
Chemical Equation
No mass is gained or lost in a chemical equation
Law of Conservation of Mass
When a substance mixes completely with another substance but no new substances are formed.
Dissolve
Balanced or unbalanced? Why or why not?
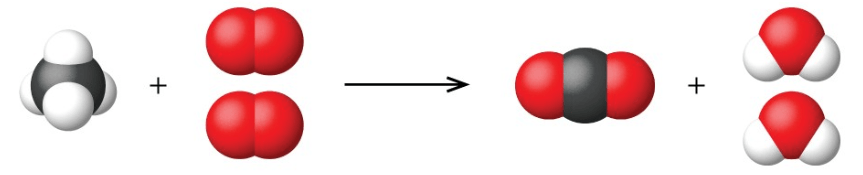
Balanced; same number of each type of atom on both sides.
4 white atoms on the left and on the right; 1 gray atom on the left and on the right; 4 red atoms on the left and on the right
Chemical reactions are taking place in the trash can. What EVIDENCE supports this claim?

A new or changed odor