Can mixtures be separated?
Yes
What is the pH for neutral solutions?
pH = 7
True or false? When a sample is divided in half, the density is also divided in half.
False, the sample may have a smaller volume and mass, but the density will always remain the same.
Name one evidence that a chemical change has occurred:
Answers vary.
Examples: fizzing, color change, forming new substances, change in temperature, change in smells, etc.
What is the density formula?
density = mass/volume
Give one example of a heterogeneous mixture.
Answers vary.
Mixture most be visually different.
Fruit salad, sand and water, salad, burger, nuts and bolts, flower arrangement, soil, trail mix, sand and iron filings
Which is an example of a pure substance?
a. salt water
b. m and m's + skittles
c. iron shavings
Answer: C. iron shavings because the sample contains pure iron.
Define: soluble.
Physical property: able to be dissolved into water.
What is a chemical change?
A chemical change is a chemical reaction or process that changes substances into new substances.
What is the volume of this sample?
Density = 4.32 g/ml
Mass - 23.9 ml
Volume = 5.53 ml
True or false? Write the true statement if it is false.
A sample of lake water; including pebbles, algae and fish, is a homogeneous mixture.
False, The lake water sample is a heterogeneous mixture.
Give me 3 examples of solutions that have a pH between 0-6.
Acids: lemons, limes, vinegar, oranges, bananas, apples, battery acid, stomach acid, HCl, saliva
Which substance has the lowest melting point?

Silver - melting point and boiling point are physical properties
Which factors will increase the reaction rate?
a. Lower temperature
b. Catalyst
c. Higher Concentration
d. Increase surface area
b. Catalyst
c. Higher Concentration
d. Increase surface area
True or false? When a sample is divided in half, the density is also divided in half.
False: The density of a substance will always remain the same, despite the sample size.
Which of these is a Homogeneous mixture?
a. Milk
b. Salt Water
c. Soda
d. Liquid Glue
a. Milk
b. Salt Water
d. Liquid Glue
NO- Soda - we can see the bubbles of CO2
What is the pH range for the following solutions: Bleach, shampoo, blood, baking soda, soaps
pH = 8-14
A block of aluminum could be pounded flat into sheet metal. Why is making sheet metal considered a physical change and not a chemical change?
The aluminum sheet metal has the same chemical composition as the block of aluminum.
Which of these are a chemical property?
a. Flammability
b. odor
c. reactivity with oxygen
d. temperature
a. Flammability - chemical property
c. Reactivity with oxygen- chemical property
Alfred investigated an antique scissor. He was trying to determine the unknown metal. Which physical property would help him make his determination?
a. magnetism
b. color
c. density
d. conductivity
Density - each element has a specific density
NO - most metals are magnetic
NO - Most metals are silver or gold
NO - Most metals are conductive
You are asked to separate the heterogeneous solution of sand and water. Describe the best way to separate the water from sand.
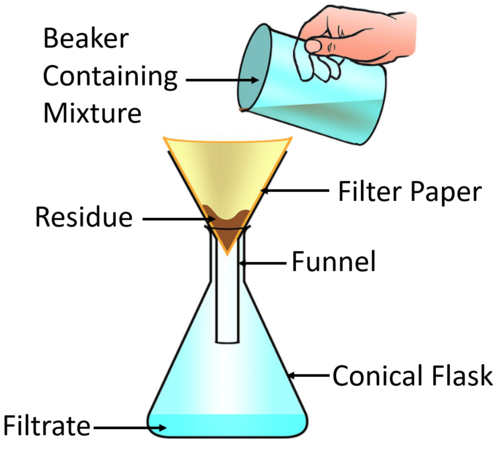
Filtration
A sample of pure substance, such as iron, will always have the same: (choose all that applies)
a. density
b. chemical composition
c. volume
d. mass
Answer : a and b
Iron will always have the same chemical composition and density, despite the size of the sample.
Not c and d because the sample may have different volumes and masses. (Different sizes)
What is the difference between observing physical properties and chemical properties?
Observing physical properties does not change the sample- ex. observing color, smell, luster.
Observing chemical properties may change the sample- ex. flammability, reactivity with acid
How does increasing temperature increase the reaction rate?
Increasing the temperature increases the kinetic energy and the molecules will move faster. Faster molecules will collide more often.
Which phase change causes a sample's density to increase?
Freezing or condensation
Molecules move closer together.