Mass
How can a mixture of iron filings and sand be separated?
By using a magnet
What does it mean when someone says "Relative" density?
the density of an item compared to water.
What does it mean when something has magnetism?
It is magnetic.
What is a conductor?
What is an insulator?
A conductor allows the flow of heat/ electrical energy.
An insulator blocks the flow of heat/ electrical energy.
What is matter?
anything that has mass and takes up space
STUFF
What are the differences between a mixture and a solution?
Mixture- easily separated, physical properties do not change
Solution- not easily separated, physical properties do change
A teacher puts 3 different colored liquids in a cup. The liquids separate into different layers by color. Then the teacher puts a ball into the cup. The ball floats in the second layer. What can be said about the density of the ball?
The density of the ball is equal to the density of the liquid in the second layer.
The density of the ball is more dense than the first layer.
The density of the ball is less dense than the bottom layer.
What tool do you use in order to test magnetism?
Magnet
Name 2 Conductors of electrical energy
Name 2 insulators of electrical energy
Iron nail, paper clip, aluminum foil... answers may vary
plastic, wood, rubber... answers may vary
What does soluble mean?
If something can dissolve.
What happens when salt water is heated?
The water is evaporated and the salt is left in the beaker.
The weight of an object determines if it will sink or float.
False.
What it is made out of.
The amount of matter in the object.
Are all metals magnetic? Give an example.
No, not all metals are magnetic.
Aluminum foil, copper (Penny)
Bonus +100
What happens to particles as they change states
They move differently and spread out as heat is added
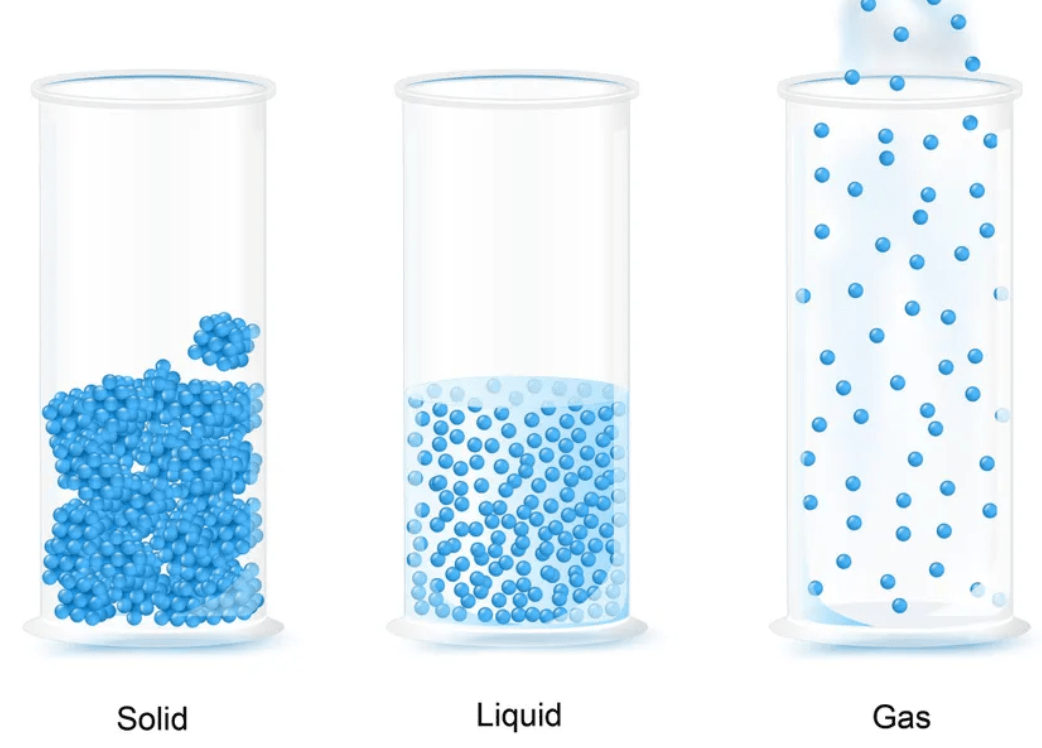
What are the physical properties of matter we have talked about?
Hint: There are 8
Mass, Volume, Magnetism, Physical State, Relative Density, Solubility, Conduct, and Insulate
Which of these might cause sugar to dissolve at a faster rate.
A. Stirring the sugar in water
B. Pouring the sugar in warm/hot water
C. Pouring the sugar a little at a time
B. Pouring the sugar in warm/hot water
Compare the relative densities of a diet soda and a regular soda based on the picture.
Diet soda is less dense than water
Regular soda is more dense than water
This is because diet soda has less mass than regular soda.
Bonus +100
What is an abiotic factor and give an example.
What is a biotic factor and give an example.
Abiotic factors are nonliving things in an environment
Example: dirt, water, sun, etc.
Biotic factors are living things in an environment
Example: Animals, plants, insects
A cardboard box contains a variety of materials—a nail, an aluminum, an eraser, and a Styrofoam cup. Based on what you know about the items’ physical properties, all of the materials could be classified as
solids.
Volume
The amount of space mater takes up
What happens to the mass and volume of a solution when the two following substances are mixed together
Salt- 5 grams
water-10 grams
The amount of matter of each substance stays the same before and after mixing.
An adult was asked to compare the masses of three objects that were all the same size but made of different materials. A balance beam was used to compare the masses.
Which list shows the balls in order from least to greatest mass?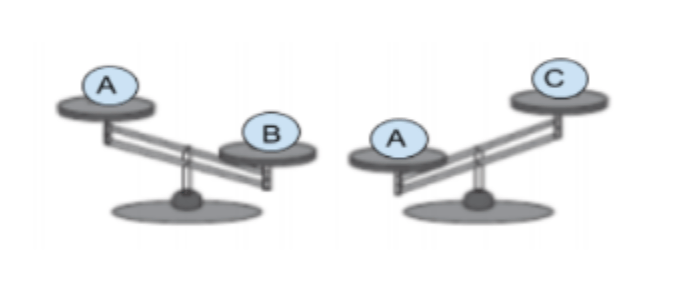
objects C, B, A
Which of the following is not magnetic?
A. Iron Nail
B. Paper clip
C. Penny
D. Binder Clip
C. Penny
Bonus +100
A. Animals respond to abiotic factors in the environment, such as snow and temperature.