Elements found on the left side of the periodic table are generally classified as ______________
What are Metals?
Between the following elements, this one is a poor conductor of heat.
F - K - Fe - Ag
F - Fluorine?
a change in matter in which the substance that makes up the matter change into other substances.
chemical change

Identify the element from this Bohr model
Calcium
A chemical reaction where heat is absorbed and the surrounding temperature falls
What is endothermic?
The temperature at which an element or compound changes from solid to liquid.
Melting Point
The metals that are the most reactive on the periodic table are found in this group
What is group 1?
What are the alkali metals?
the ability of a substance to be hammered or rolled into sheets.
malleability
I am an element with a full outer shell of electrons, I have as many neutrons as I do protons and I reside often in glass tubes. Who am I?
Who/What is Neon?
Balance the following:
______ CO2 + 6 H2O -----------> C6H12O6 + ____ O2
What is 6, 6
Mass per unit volume of an object as compared to pure water.
What is Density?
Of the following choices, these are signs of a chemical change:
-change in color, size and shape
-change in color, produces gas and heat
-change in states of matter (freezing, melting, boiling)
change in color, produces gas and heat
Given 100 centimeters, would equal __________ kilometers
What is .001 kilometers
a positively charged particle in the nucleus of an atom.
proton
Baking bread in an oven is an example of a chemical or physical reaction?
What is a chemical reaction?
Rust forms when iron is exposed to oxygen and moisture for a long period of time. Rust indicates this type of chemical reaction.
Chemical reaction
new substances formed in a chemical reaction
products
Material is a poor conductor of heat/electricity, it has a high number of valence electrons and is not solid.
What is a non-metal?
The measurement that shows the amount of space something takes up is known as
What is volume?
Number of protons, neutrons and electrons in Potassium
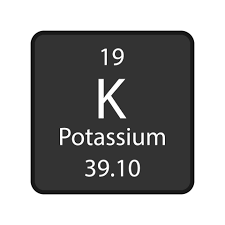
19 - protons
19 - electrons
20 - neutrons
An ionic bond occurs between calcium and fluorine. After the reaction, the chemical compound produced has the formula__________
What is CaF2
A reaction happens: zinc metal is added to hydrochloric acid and produces the compound zinc chloride and releases hydrogen gas. What type of reaction is this
What is single - replacement?
Measuring this will tell if a reaction is endothermic or exothermic
What is temperature?
In the production of glucose, C6H12O6 all three atoms are ______________ electrons and therefore forming a/an _______________ bond.
What is sharing, covalent
a change in the size, shape, form, or state of matter that does not change the identity of the matter.
physical change