In a chemical reaction, is it possible to change the amount of the products by changing the amount of the reactants? Why or Why not?
Yes if you increase or decrease the amount of the reactants, it will increase or decrease the amount of the products.
A chemical reaction produces two new substances, and each product has a mass of 25g. What was the total mass of the reactants?
What is 50g
How many molecules of water are formed in the reaction: 2 H2O2 🡪 2 H2O + O2?
What are two molecules of water (H2O)?
A chemical reaction occurs, one of the reactants has a mass of 35g. The product side is 100 g. What is the mass of the other reactant?
What is 65g
A teacher demonstrates a chemical reaction for the class. Which of these would NOT show evidence that a chemical reaction occurred? a) change in color b) change in shape c)formation of a gas
What is change in shape
What increases the rate of a chemical reaction?
What is increasing temperature and a catalyst?
When salt is mined, machines scrape the mine walls and change salt rocks into very tiny salt crystals. What happens to the mass of 1 kilogram of salt rocks when the rocks change into very tiny salt crystals?
What is the total mass stays the same?
Students combined baking soda and vinegar to demonstrate a chemical reaction. What would indicate that a chemical reaction occurred?
What is the formation of bubbles/gas?
Describe the Lewis Dot Diagram for Oxygen (which has 6 valence electrons)
What is the O symbol in the middle, two dots on the top and right of the O, and one dot on the left and bottom?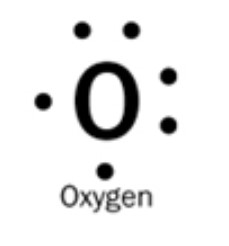
In a reaction with vinegar and baking soda, can you continue to add more and more of one reactant and expect to get more and more product? Why or why not?
No, if you continue to add more of only one reactant, it will eventually run out of the other reactant to react with.
When you combine vinegar and baking soda, a gas is produced. Why is the gas considered evidence that a chemical reaction occurred?
Gas is evidence because it was not part of the reactants. The reactants vinegar and baking soda when mixed, produce a gas which you can see by the formation of bubbles.
What is the difference between an ionic and covalent bond?
What is atoms transfer electrons in an ionic bond and share electrons in a covalent bond?