has a definite shape and volume
solid
HOW do we balance a chemical reaction?
By changing the coefficients in front of the products and reactants
What is the correct name for HgCl
What is Mercury (I) Chloride
another word for a chemical change
chemical reaction
anything that has mass and takes up space
matter
What does the Atomic number mean?
The number of Protons, which also equals the number of electrons for a neutral atom.
Being shiny, malleable, and conductors are properties of this class of elements.
metals
A covalent bond will always form when these two types of elements react.
Two non-metals
when a substances changes its state
change of state
Is the following equation balanced
___ LiCl → ___ Li + ___ Cl2
No
What is the correct name for As2S5
What is Diarsenic pentasulfide
CO2 → C + O2
Decomposition
Matter that is physically combined, but not chemically combined, usually a liquid and a solid, where one form is no longer visible
Solution
Groups on the periodic table are columns or rows?
columns
Reason Noble gases are stable.
What is a full outer energy level?
Daily Double
What charge does copper have in the ionic compound CuCl2?
2+ (Copper (II))
does not have a definite shape but has a definite volume
liquid
___ Zn + ____ H3PO4 → ___ Zn3(PO4)2 + ____ H2
___ Zn + ____ H3PO4 → ___ Zn3(PO4)2 + ____ H2
What is the correct formula for Lead(IV) Oxide
What is PbO2
NaCl + AgNO3 → NaNO3 + AgCl
Double Replacement
Compound
How many Neutrons are in a Uranium atom? 
238-92=146 neutrons
Metals form this type of ion.
What are cations (+ ions). They GIVE valence electrons.
A positively charged ion is called a/an _____.
cation
does not have a definite shape nor volume
gas
daily double
___ Al + ____ CuCl2 → ____ AlCl3 + ____ Cu
2 Al + 3 CuCl2 → 2 AlCl3 + 3 Cu
What is the correct name for Ga(ClO3)3
What is Gallium Chlorate
2 C2H2 + 5O2 → 4 CO2 + 2 H2O
2 C2H2 + 5O2 → 4 CO2 + 2 H2O
a blend of two or more kinds of matter each of which retains its own properties
mixture
The elements in group 17 are known as this.
halogens
Silicon, Boron, and Tellurium are examples of this class of elements.
What are metalloids?
What type(s) of bonds are shown in the compound below?
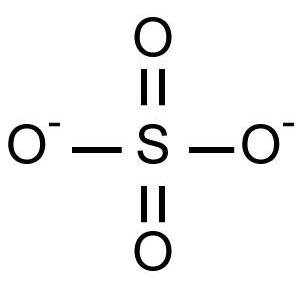
2 single bonds and 2 double bonds
a physical state of matter where atoms lose most of their electrons
plasma
Balance the following equation
_ MnO2 + __ HCl → __MnCl2 + __ Cl2 + ____H2O
1 MnO2 + 4 HCl → 1 MnCl2 + 1 Cl2 + 2 H2O
What is the correct formula for Cobalt (III) Hydroxide
What is Co(OH)3
Pb(NO3)2 + Mg → Pb + Mg(NO3)2
Single Replacement
Which of the following is a pure substance?
a. The Air you breathe
B. The Brass in your trumpet
C. a Diamond
D. Light
A diamond
daily double
The modern periodic table organizes elements according to this.
What is increasing atomic number?
What trend increases as you move from the middle of the periodic table to EITHER the left side or the right side in a period?
How reactive the elements are! Period 1 and Period 7 are VERY reactive!
What type of bond was formed in the following:
NH4F?
Ionic
A new element is found with an atomic number of 119.
Where would you place it on the periodic table and what type of material would it be called? What would it's chemical properties be?
It should be in period 8 and group 1. So it should act like an alkali metal (be explosive in water and VERY reactive).