What all matter is made up of
What are elements?
Originally thought to be the four elements that make up matter
What are earth, air, fire, and water?
Describes most of the elements
What is rare?
The temperature at which liquid changes into a gas
What is the boiling point?
The amount of space an object takes up is called
What is volume?
The center of an atom
What is the nucleus?
The modern periodic table arranges the elements by this
What are their properties?
98% of Earth's crust is made up of only 8 elements, name three
What are oxygen, silicon, aluminum, iron, calcium, sodium, potassium, and magnesium? (name 3 to get the points)
Can be measured or detected by the senses
What are physical properties?
The change of state from liquid to gas
What is vaporization? (evaporation will also be accepted)
The nucleus contains two kinds of particles
What are protons and neutrons?
Elements in the periodic table are arranged by this
What is their atomic number?
This element is the building block of matter in living things
What is carbon?
The measure of how much of one substance can be dissolved in another
What is solubility?
A mixture made of two or more evenly distributed substances
What is a solution?
Move in space around the nucleus
What are electrons?
The atomic number of an element is determined by this
What is the number of protons in the nucleus?
Earth's atmosphere is made of these two elements
What are oxygen and nitrogen?
Compound that typically accepts hydrogen ions
What is an base?
The process in which a solid changes directly to a gas
What is sublimation?
When elements combine with other elements
What are compounds?
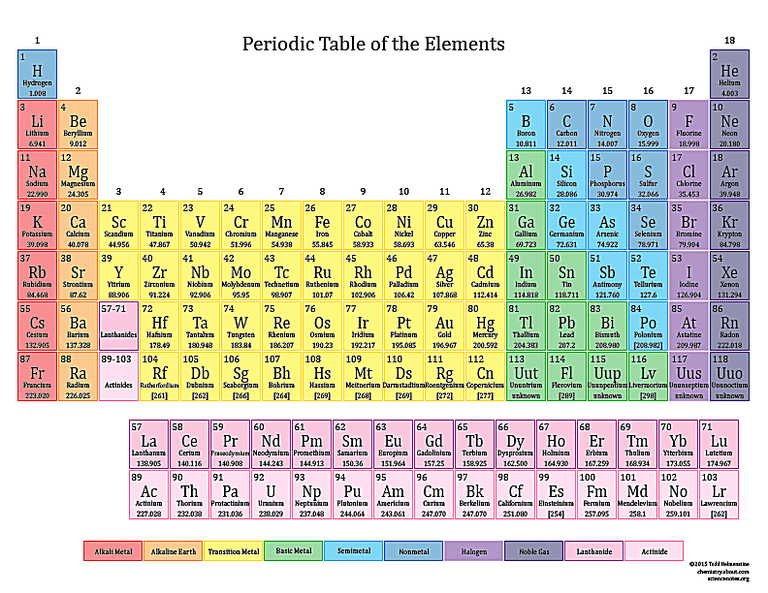
According to the Periodic Table Potassium is in this group
What is metal?
What are hydrogen, nitrogen, carbon, oxygen, calcium (name at least 3)
Forms when a strong acid reacts with a strong base
What is salt?
A substance that enters into and is altered through a chemical change
What is a reactant?