Which subatomic particle has a neutral charge?
Neutron
The horizontal rows of the periodic table are called?
Periods
Chemical reactions that release energy are known as..
Exothermic
How many valence electrons does Aluminum have?
3
What types of elements are present in covalent bonds?
Nonmetals and Nonmetals
What are the electrons called in the outermost shell of an atom?
Valence Electrons
What is the name of the periodic family with a full octet of valence electrons?
2
What types of elements are present in ionic bonds?
Metals and nonmetals
How many electrons does lithium (Li) have?
3
Moving from left to right on the periodic table, does atomic radius increase or decrease?
Decrease
Endothermic
How many electrons are present in a triple bond?
6
In what type of bond are electrons shared?
Covalent
Positive
Highest ionization energy, K or Br?
Br
What type of reaction occurs when a log burns in a fireplace?
Exothermic
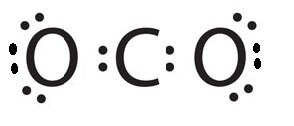
Is this the correct LDS for CO2?
No
What type of bonding does Potassium Chloride (KCl) do?
Ionic
How many neutrons does francium have?
136
Which element has the lowest electronegativity? Br or K?
In which type of reaction will the energy of the products be higher than the energy of the reactants?
Endothermic
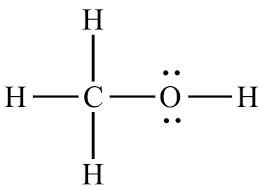 Is this the correct lewis structure for CH3OH?
Is this the correct lewis structure for CH3OH?
Yes
What value do I use to calculate Percent Ionic Character? Atomic radius, ionization energy or electronegativity?
Electronegativity