Columns (up and down) within the periodic table are called _____
Families
What are three signs that a chemical change has occurred?
- Produce Light
- Produce heat
-Produce an odor
- Change in color
- Create a precipitate
Give an example of an alkaline earth metal
Be, Mg, Ca, Sr, Ba, Ra
If atoms give and take away electrons, which type of bond is being studied?
Ionic
A _____ is a positively charged particle in the nucleus that determines the element's identity.
Proton
Provide 2 examples of physical change and 2 examples of chemical changes
Multiple answers
A physical change will change shape or size without creating a new substance
A chemical change will create new substances
Draw Bohr's model of Carbon

What is the ultimate goal when atoms form a bond?
To reach 8 valence electrons, either by filling or emptying their outer shell.
A _____ is a pure substance made from two or more elements chemically combined in fixed proportions.
Compound
A mystery substance has a mass of 171g and a volume of 15 cm3. Based on the table provided, what substance is being observed?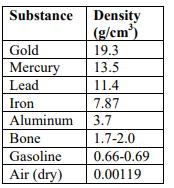
Lead
Why does most of the mass of an atom exist in the nucleus?
Protons and neutrons are x2000 larger than electrons, and both of those subatomic particles are located in the nucleus.
Draw the lewis dot for the following elements: Mg and F

_____ are atoms of the same element that have the same number of protons but different numbers of neutrons.
Isotopes
Identify the following matter as a pure substance or a mixture.
Then, identify it as an element, compound, homogeneous mixture, or heterogeneous mixture:
Jello
Mixture
Homogeneous Mixture
What is the atomic number of Chlorine?
17
Which atoms do ionic bonding?
Metal and non metal
_____ is the most dangerous form of radiation
Beta
Identify the following matter as a pure substance or a mixture.
Then, identify it as an element, compound, homogeneous mixture, or heterogeneous mixture:
Copper Sulfate (CuSO4)
Pure substance
Compound
How many protons, neutrons and electrons are in Oxygen?
8 Protons, 8 Neutrons and 8 Electrons
Name the following: P Br3
Phosphorous tribromide
_____ is a force that resists motion between 2 surfaces
Friction
A mystery substance has the dimensions seen below and a density of 1.88 g/cm3. What is the mass of this substance in grams?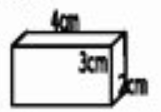
45.12 g
Fill in the 4 blanks with the correct numbers. You have 3 minutes
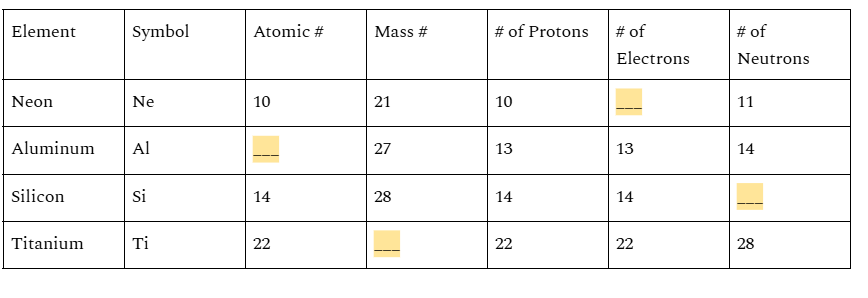
Blank 1= 10
Blank 2= 13
Blank 3= 14
Blank 4= 50
Ionic or covalent: MgO
Ionic