Carbon-14 undergoes beta decay. What two products are produced?
Nitrogen-14 and an electron.
Name this polyatomic ion:
OH-
Hydroxide
What is the density of a solid that has a mass of 48g and a volume of 5.33 cm3?
9 g/cm3
What is the atomic mass of Oxygen?
16 amu
What term identifies the homogeneous mixture that forms when sugar is dissolved in a glass of hot water?
Solution
Thallium-202 undergoes gamma decay. What two products are produced?
Thallium-202 and a photon.
What is the chemical name of Cl2O7?
Dichlorine heptoxide
True/False:
Brass is a pure substance?
False
How many acetate ions would ionically bond to a single ammonium ion?
1
How many non-metals are listed on the left side of the periodic table?
1 - Hydrogen
What fraction of a radioisotope remains after 4 half-lives have elapsed?
1/16th
Balance:
___CO2 + ___H2O --> ___C6H12O6 + ___O2
6CO2 + 6H2O --> C6H12O6 + 6O2
An unknown substance that is cubic in shape has a mass of 28 g and each side of the cube is 3 cm in length. Will the substance float or sink in water?
Float
Which 7 elements typically exist diatomically?
H, O, F, Br, I, N, Cl
Draw the dot structure for Carbon Dioxide
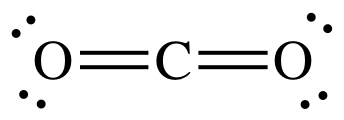
Carbon-14 has a half-life of 5730 years. How much carbon-14 would have been present in an organism when it died if its remains are determined to be 17,190 years old and it currently has 57 grams of carbon-14?
456 grams
What type of reaction is this?
Lead reacts with iron (II) sulfate to produce lead (II) sulfate and iron
Single displacement
Calculate the mass of a liquid with a density of 2.5 g/mL and a volume of 15 mL.
37.5 g
Is the compound NH4I
Covalent, Metallic, or Ionic?
Ionic
Potassium iodide reacts with bromine gas to produce what two products?
(You may give the names or chemical formulas but they have to be 100% correct)
Potassium bromide and iodine
KBr + I2
The half-life of a radioisotope is known to be 56,905 years. If you started with 100 grams of the radio isotope, how much would remain after 14,750 years?
83.6 grams remain
Name the following compound:
H2PO4-
Dihydrogen phosphate ion
A graduated cylinder with water is used to measure the displacement of a rock. When the rock is added the water level rises from 50 mL to 78.4 mL. The rock has a mass of 85.2 g. What is the density of the rock?
Silver Oxide has a chemical formula of Ag2O
What is the oxidation number of silver in this ionic compound?
+1
(Silver is ALWAYS +1)
How many moles of carbon dioxide would be present in the balanced combustion reaction of acetylene (C2H2)?
4 Moles CO2