What is the phase change called in which a gas turns into a liquid?
What is Condensation?
This is anything that has mass and takes up space.
What is matter?
This particle determines an element's identity.
What are protons?
This is an ion with a positive charge.
What is a cation?
Ionic bonds are formed between these two types of elements.
What are metals and nonmetals?
Chemical reactions must obey this law.
What is the Law of Conservation of Mass?
Which state(s) of matter have a definite volume?
What are solids and liquids?
Boiling point and melting point are examples of this type of property.
What are physical properties?
These types of electrons are used in chemical bonding.
What are valence electrons?
This family is the most reactive metals.
What are alkali metals?
This type of bond shares electrons.
What are covalent bonds?
What are the products in this chemical reaction?

What are O2 and C6H12O6?
This is the property of a fluid resisting to flow.
What is viscosity?
Granite has a density of 2.59 g/cm3. If it has a volume of 237 cm3, then this is its mass (correct units need to be included to get the points).
What is 613.83 grams?
This is the charge of the nucleus.
What is positive?
This family has 7 valence electrons.
What are Halogens?
Diphosphorus pentoxide has this many oxygen atoms.
What is 5?
The following chemical reaction is what type of reaction?

What is Double Replacement (Displacement)?
The type of process in which energy is released.
What is exothermic?
Brass is an example of this type of mixture.
What is homogenous?
This is the element that's shown below.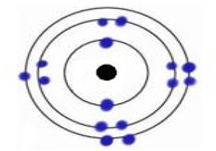
What is Phosphorus?
This family is considered inert (nonreactive).
What are noble gases?
What is MgCl2?
What will always be the products in a combustion reaction?
What are CO2 and H2O?
This is what happens to the particles when a substance melts.
What is speed up and spread apart?
If a substance has a volume of 14 mL and a mass of 13.2 grams, then this is its density (correct units need to be used to get the points).
What is 0.94 g/mL?
This is the number of neutrons in Barium-127.

What are 71 neutrons?
These are the inner transition metals.
What are Lanthanides and Actinides?
This is the chemical formula for the compound Dinitrogen trioxide.
What is N2O3?
This is the number of oxygen atoms present in the reactants of the following equation:
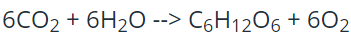
What is 18?
This is the mass of H2.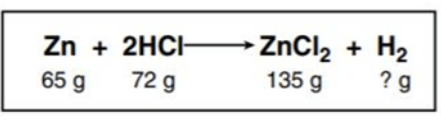
What is 2 grams?
This substance will float in water but sink in kerosene.

What is benzene?
This is what protons and neutrons are made of.
What are quarks?
Iron is in this block.
What is d-block?
This is the name of the compound Na2O.
What is Sodium oxide?
The following coefficient must be added in front of Al2O3 in order to balance this equation.

What is 2?