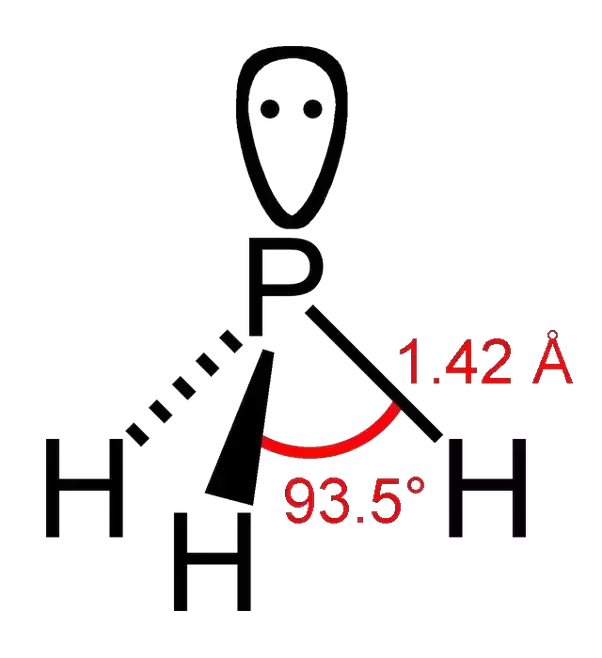
Draw the lewis structure of phosphorus trihydride
Based on the following balanced chemical equation, what is the mole ratio for N2 to O2?
N2 (g) + O2 (g) --> 2NO (g)
1:1
At the end of a chemical reaction, which reactant is still present in the reaction vessel?
excess
What is the equation to calculate percent yield of a certain product?
percent yield = (actual yield)/(theoretical yield) x 100
True or false? According to the law of conservation of mass, the mass of reactants is the same as that of the products of a chemical reaction
True
What is the electron domain geometry and molecular geometry of hydrogen disulfide?
EDG: tetrahedral
MG: bent
First, balance the chemical equation for the combustion of C3H8:
C3H8 (g) + O2 (g) --> CO2 (g) + H2O (g)
What is the mole ratio of O2 to CO2? Write two conversion factors.
(5 mol O2)/(3 mol CO2), (3 mol CO2)/(5 mol O2)
How many grams of Co2S3 can be produced from the reaction of 250. g of Co and 250. g of S?
The chemical equation is:
2Co (s) + 3S (s) --> Co2S3 (s)
454 g
How is the actual yield determined (in the calculation for percent yield)?
Through experiment
What is the sign (+ or -) of delta(H) for endothermic reactions?
positive
Specify the following information for a molecule of carbon dioxide:
# bonded pairs:
# lone pairs:
Bond angle:
2 bonded pairs,
0 lone pairs,
180 deg.
How many moles of oxygen are needed to react with 0.360 mol of ethanol (C2H6O)?
The chemical equation for this reaction is:
C2H6O (g) + 3O2 (g) --> 2CO2 (g) + 3H2O (g)
1.08 mol
If 50.0 g of C3H8 and 150. g of O2 react, the LR is:
The chemical equation is: (unbalanced!)
C3H8 (g) + O2 (g) --> CO2 (g) + H2O (g)
O2
The chemical equation is:
2C2H6 (g) + 7O2 (g) --> 4CO2 (g) + 6H2O (g)
74.0%
The limiting reactant is ALWAYS the reactant with the fewest moles
False
True or false: the molecular geometry is the same for these two molecules: Draw the lewis structure for each to support your answer
methane (CH4)
PCl5
False
How many grams of carbon dioxide are produced when 92.0 g of ethanol (C2H6O) reacts?
The chemical equation is:
C2H6O (g) + 3O2 (g) --> 2CO2 (g) + 3H2O (g)
176 g
Consider respiration, one of the most common chemical reactions on earth:
C6H12O6 +6O2 --> 6CO2 + 6H2O + energy
How many moles of carbon dioxide forms when 25 g of glucose reacts with 40 grams of oxygen?
0.83 mol
If 23.3 g of galactose reacts to give 26.3 g of CO2, what is the percent yield of CO2?
The chemical equation is:
C6H12O6 (aq) + 6O2 (g) --> 6CO2 (g) + 6H2O (l)
76.9%
Where is "heat" placed in a chemical reaction for exothermic reactions? (reactants side or products side)
products side
Which molecule has a multiple bond? N2 of F2? Draw the lewis structure for each
N2
When 25.0 g of N2 reacts, what is the theoretical yield of NH3 (in grams)?
First, balance the equation:
N2 (g) + H2 (g) --> NH3 (g)
30.4 g
Consider respiration, one of the most common chemical reactions on earth:
C6H12O6 +6O2 --> 6CO2 + 6H2O + energy
25 g of glucose reacts with 40 grams of oxygen...how many moles of excess reagent are leftover?
0.42 mol
If 25.0 g of O2 reacting with sufficient H2S produces 18.6 g of SO2, what is the percent yield of SO2?
First, balance this equation:
H2S (g) + O2 (g) --> SO2 (g) + H2O (g)
55.7%
Is the following reaction endothermic or exothermic?
CaCO3(s) + 556 kJ --> CaO(s) + CO2(g)
endothermic