Describe what happens to the particles of an ice cube as it changes phases (from solid to a gas). Your answer needs to take in account the motion, spacing and temperature as you describe each transition. You may also use drawings along with your description.
An ice cube will begin to change phases as it takes on heat energy. The particles will begin to change from tightly packed and slow moving in its solid phase to its liquid phase. This liquid phase will be characterized by the arrangement of particles being loosely packed and having more motion. Finally, particles will be much further apart and move quickly and randomly as heat energy is applied causing the phase to go from a liquid to a gas.
Inez has two beakers that contain ethanol. One beaker contains 150 mL of liquid. The other beaker contains 300 mL of liquid. What can Inez expect to observe when she compares the properties of the liquid in the beakers. What properties should be the same in the two beakers
Inez could observe that the two beakers have the same boiling and melting points.
Students should understand that there are seven properties that are size independent: density, boiling/melting point, state of matter, electrical conductivity, solubility, magnetism
Which of the following statements is an example of a prediction?
A. The metal was shiny and brittle.
B. The sugar had a mass of 15 grams.
C. The type of sugar will affect the respiration rate of yeast.
D. The amount of oxygen produced by the plant decreased as the amount of light decreased.
The correct answer to the question is letter C, the type of sugar will affect the respiration rate.
Even though atoms are much too small to see, experiments have helped scientists learn about the subatomic particles that compose atoms. The current model of an atom has a nucleus in the center surrounded by an electron cloud. Which of these parts of the atom has the greatest mass? Explain your reasoning.
The nucleus of an atom has the greatest mass because it contains the neutrons and protons. The electron cloud contains electrons which are so small their mass does not account for much of the total mass.
Leslie adds 10g of zinc to 120g of hydrochloric acid. The mixture begins to bubble. After the reaction, she takes the mass of the resulting mixture which was 115 g. Why is there a change in mass?
A. A gas formed and escaped into the air
B. The hydrochloric acid evaporated during the experiment.
C. Mixtures always are less massive than the starting materials
D. Mass was destroyed during the reaction.
A gas formed and escaped into the air Correct, the missing mass has escaped into the air as the gas that was produced from the bubbles in the reaction.
Liam is constructing a model showing how a sound wave travels through a solid, liquid and gas. He understands there is a relationship between the speed of the wave and the type of medium (or material) it travels through. Based on your understanding on the different states of matter, how would a sound waves speed change between a gas, liquid and sold? Why does the speed change?
Sound waves travel the fastest through a solid then a liquid and the slowest in a gas. Sound waves needs to vibrate particles or molecules to travel. Particles or molecules in a solid are closely packed together and therefore the sound wave will be able to move the fastest through a solid material. As particles move further apart, the slower the wave will travel. Particles in a liquid are further apart than a solid and particles in a gas are even more spread apart.
Edwardo wants to see at what temperature the water would boil. Below is a chart of his finding
Substance Amount of substance Type of container Time taken to boil Boiling temp. in degrees F
Water 350 ml Teflon pot 3 min 212
Water 775 ml Glass pot 4 min 212
Water 850 ml Metal pot 5 min 21
What conclusion can be drawn about his findings?
Water has the same boiling point regardless of the amount of substance. Water is a size independent property.
Mario wanted to test the effect of temperature on the ability of sugar to dissolve in water. He began his experiment by labeling three beakers A, B and C. He poured 100 milliliters (mL) of 5 degrees Celsius (℃) water into beaker A, 100 mL of 20 ℃ water into beaker B, and 100 mL of 50 ℃ water in beaker C. He added 10 grams (g) of sugar to each beaker and stirred for 1 minute.
Identify the independent (test) variable and the dependent (outcome) variable for this experiment
The independent variable is the temperature of the water. The dependent variable is amount of sugar that will be dissolved.
Using information from the periodic table, draw a diagram of the of the Magnesium (Mg)
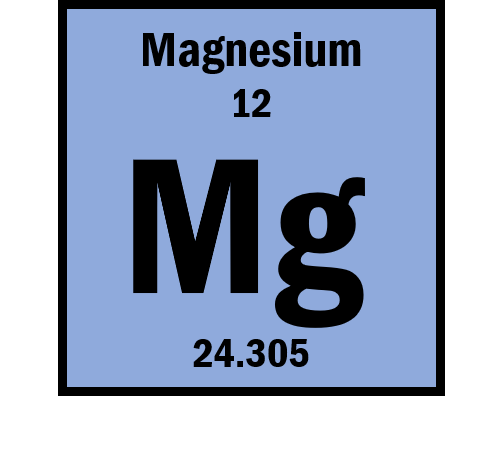
Leigh is preparing popcorn for her friends. She removes a stick of butter from the refrigerator and cuts a fourth of it from the rest. Leigh remembers her lesson in school today about the conservation of mass that occurs during state changes. She wants to test what she learned, so she measures the mass of the butter she will melt. Its mass is 35 g. Then, she places the butter in the microwave and melts it.
What should the mass of the liquid butter be once it is melted?
A. 70 g
B. 40 g
C. 15 g
D. 35 g
35 g Correct, mass cannot be created or destroyed so the mass of the butter should remain the same if it doesn’t spill.
Sincere wants to make a model of what happens to the particles in lava as they cool after a volcano eruption. He constructs two models; the first model is of molten rock as it escapes the volcano, and the second model is of solid igneous rock that has cooled at the base of the volcano. How would the particles in his first model differ from the particles in the second model?
A. The particles in the first model should only vibrate in place and the particles in the second model should move freely in all directions
B. The particles in the first model should move freely while sliding past each other and the particles in the second model should vibrate in place
C. The particles in both models should be able to slide past each other
D. The particles in the first model should move freely in all directions and the particles in the second model should not move at al
B. The particles in the first model should move faster while sliding past each other and the particles in the second model should vibrate in place Correct, liquid particles slide past each other and particles in solids vibrate in place
Carlton and a classmate were given three beakers that each contained a different amount of ethanol. The classmate was unclear as to how they could have the same boiling points. Write an explanation as to why the boiling point stays the same for each beaker?
Boiling point is a size independent physical property. The boiling point of a substance will remain constant regardless of size
Amal is designing an experiment to test the effect of varying amounts of light on the rate of photosynthesis. He hypothesizes that the amount of oxygen produced is related to the amount of light a plant receives. He plans to use four plants of the same species and size. He will expose three of the plants to light for different lengths of time. The fourth plant will receive no sunlight at all. He will measure oxygen production for all four plants. The fourth plant will serve as the control for the experiment. What is the purpose of a control in an experiment?
The purpose of the control is to serve as a comparison for the experimental portion of the experiment
What is the smallest part of an element that still retains properties of that element?
A. Molecule
B. Mixture
C. Compound
D. Atom
Atom Correct, an atom is the basic unit of matter. An atom of an element is the smallest unit that will still retain all properties of the element. Anything smaller would be subatomic and not an element anymore.
Regan has an empty glass cup. It has a mass of 0.3 kg. She drops the cup on the ground, and it shatters into several pieces. If she collected all the pieces of the cup, how much mass would all the pieces have combined?
A. There is no way to determine the mass of the shattered glass
B. Less than 0.3 kg
C. Exactly 0.3 kg
D. More than 0.3 kg
Exactly 0.3 kg Correct, mass cannot be created or destroyed so the mass would remain the same.
Sami pops a helium balloon at a birthday party. What will happen to the particles of helium that were in the balloon?
a. The particles will disappear.
b. The particles will move together.
c. The particles will spread out evenly within the room.
d. The particles will stick together and sink to the floor.
c. The particles will spread out evenly within the room. *
The _______ of a substance is the amount of that substance that can dissolve in a certain amount of a given solvent.
A. Boiling Point
B. Density
C. Solubility
D. Chemical Reactivity
Solubility Correct, solubility is the amount of a substance that can dissolve in another substance.
Chandra has three pieces of metal with equal masses. Each piece is the same color but has a different shape. She suspects that all three pieces are made of the same element, so she decides to measure the volume of each piece. Develop a hypothesis based on these materials?
Chandra should know that density is a physical property that can be used to identify an element. Her hypothesis can be if the volumes of each piece is also identical, then the three pieces of metal all have the same density.
Edward is constructing a model of a neutral carbon atom using Styrofoam spheres. He is deciding where to place the electrons on his model. He knows from the Periodic Table that he needs six electrons for his model. Which description would be an accurate description of where Edward should place the electrons in his model
A. He should place the electrons in the center of the atom within the nucleus and label them with a positive charge.
B. He should place the electrons on the outside of the nucleus and label them with a negative charge.
C. He should place the electrons in the center of the atom within the nucleus and label them with a neutral charge.
D. He should place the electrons on the outside of the nucleus and label with a positive charge.
He should place the electrons on the outside of the nucleus and label them with a negative charge. Correct, electrons are located in the electron cloud with a negative charge according to the current atomic model
There are characteristics that differentiate a chemical change from a physical change. Which statement describes a physical change?
A. Iron combines with oxygen to form rust.
B. Lead is heated and becomes melts into a viscous liquid.
C. Sodium metal reacts explosively with oxygen
D. Calcium is added to acid and hydrogen gas is produced
Lead is heated and becomes melts into a viscous liquid. Correct, melting (or any phase change) is a physical change.
Raul and Bonnie are trying to classify several objects that they found during a field trip to the beach. They collected a sample of seawater, a piece of driftwood, and several smooth pebbles. What property does the driftwood share with the pebbles that it does NOT share with the water?
a. Its particles move freely in all directions.
b. Its particles vibrate but are locked in place.
c. Its volume does not depend on its container.
d. Its volume will change in different containers
c. Its volume does not depend on its container.
What will happen to the molecules of a substance when heat is continuously added to a liquid?
The substance will reach it boiling point and eventually evaporate
It is okay to pick up broken glass with your bare hands as long as the glass is placed in the trash can.
True or false
False notify the teacher to pick up broken class to dispose of glass properly.
Automatic Points for picking category
add 500 points !!!!
Ella is planning an experiment. She wants to see how fast milk will sour at different temperatures. She places a glass of milk under a warming lamp (35°C) and leaves another at room temperature (25°C). She checks the milk every three hours. Which outcome is most likely?
A. Neither glass of milk will sour.
B. Both glasses of milk will sour at the same time
C. The milk under the warming lamp will become sour more quickly.
D. The milk left at room temperature will become sour more quickly.
The milk under the warming lamp will become sour more quickly. Correct, the increased temperature will cause the chemical reaction of souring milk to occur at a faster rate.