Polarity confused Mr. Giles, what one way to remember it using poles?
North Pole and South Pole just like a Positive Pole and Negative pole for electrons!

Polar
Have a pole (a side that is negative or positive - usually it's both!)
All molec_____ have this f_rce
All molecules have this force
Dipole Dipole is this kind of strength for molecule attraction
Middle or medium strength for dipole dipole
The strong__t bond is hydrog___ bond____
The strongest bond is hydrogen bonding (strongest of all 3 types!)
Non polar means:
There are no poles so everything is equal with the electrons.
Think No poles = non magnet
Polarized
This force is the weak___ of the thr__ we learned about yesterday!
This force is the weakest of the three we learned about yesterday!
What does di and pole mean again?
di = 2
pole = sides/ locations
dipole= (one side is positive and one side is negative, 2 poles!)
If Hydrog____ pairs with N,O , or F (which are __________) it makes hydrogen bonding! They are all next to each other at the t_p of the periodic table.
If Hydrogen pairs with N,O , or F (which are nitrogen, oxygen or flourine) it makes hydrogen bonding!
They are all next to each other at the top of the periodic table.
Polarity can be perm____________ or tempor__________
permanent or temporary
If I got fired and some people supported me and were mad and other people were glad I got fired and hated me, we could say that I was a pol________ figure.
Polarizing figure, because people either hated me or loved me but the feelings would be strong on both sides. Who are there polarizing figures?
Disperse, dispersion means:
to scatter. If security says to disperse the crowd, what are they saying?
dipole basically means that (again)
there are 2 sides of attraction with the electrons, one side that is more positive and another side that is more negative
The bonds are stronger because the electrons are __________
The bonds are stronger because the electrons are closer to the nucleus!
If polarity is permanent or temporary, that means that the magnet on a molecule could also be:
that means that the magnet on a molecule could also be permanent or temporary
The room was split into 2 parts (because of the prefix Di)
Your goal is to find the high___ for____ a molecule has and lab___ it.
Your goal is to find the highest force a molecule has and label it.
This example shows what?

H and Cl in a dipole dipole attraction, what's happening? How do we know?

This is an example of hydr_____ because..... it has an ___ and an ___ in it!
This is an example of hydrogen bonding because..... it has an H and an O in it!
Polarization in politics means
That we are split into 2 groups like democrats and republicans (and people might not like working together).
Dipole (you will see this twice)
Di = (means) tw__
pole = s_de/l_cation (posi___ and nega___)
Dipole (you will see this twice)
Di = (means) two = 2
pole = sides/locations (positive and negative!)
All molecules can m__ve so because they all sc____ and m___ they have this for_____
All molecules can move so because they all scatter and move they have this force.
(not in your notes) How many dipole do I have in this picture? 1 dipole, or 2 dipole dipole?
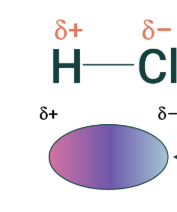
1 dipole
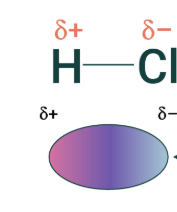
Practicing this can help you with your form___ summ and jeo
Practicing this can help you with your formative summative and jeopardy!

