Pyrohotite is a type of mineral that is able to attract certain types of metals like copper, nickel, and iron. What property does this mineral have?
A. density
B. magnetism
C. reflectivity
D. conductivity
What is magnetism?
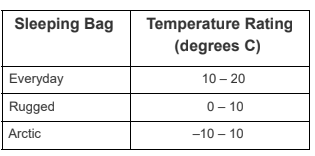 The chart shown appears in a catalog of camping equipment.
The chart shown appears in a catalog of camping equipment.
The difference between the Arctic sleeping bag and the Rugged sleeping bag is most likely that the Arctic slepping bag is _________.
A. designed to last longer.
B. made of materials that insulate better.
C. designed for home as well as camping.
D. made of materials that provide better cushion.
What is made of materials that insulate better?
A student observes a small iron ball and records its properties, as shown.

The student classifies the iron ball as a solid. Which statement explains why the student is correct or incorrect?
A. The student is correct because a solid has a fixed shape and volume.
B. The student is correct because only a solid has mass and occupies space.
C. The student is incorrect because a solid does not retain its mass and volume.
D. The student is incorrect because a solid takes the shape of the container it is placed in.
What is the student is correct because a solid has a fixed shape and volume?
Mrs. Johnson's class is testing electrical conductivity of different substances. Which of the following substances, when tested, will be an electrical insulator?
A. rubber band
B. paper clip
C. nail
D. fork
What is a rubber band?
Which of the following substances would be a good electrical conductor?
A. iron nail
B. rubber ball
C. cotton sweater
D. wooden spoon
What is an iron nail?
The table contains information about metals and nonmetals.
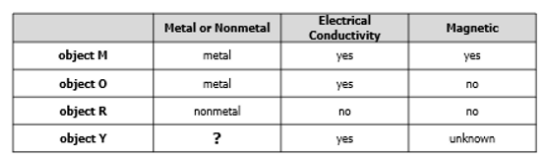
A. Object Y is most likely a nonmetal because it can conduct electricity.
B. Object Y is most likely a nonmetal because it is magnetic.
C. Object Y is most likely a metal because it can conduct electricity.
D. Object Y is most likely a metal because it is magnetic.
What is object Y is most likely a metal because it can conduct electricity?
A student fills a bowl with liquid and places one marble and one cork in the bowl as shown.
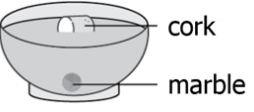
The student concludes that the marble is more dense than the cork. Is the conclusion correct, and why?
A. No, the cork floats making it more dense than the marble.
B. No, the marble sinks making it more dense than the cork.
C. Yes, the marble is more dense than the liquid and sinks while the cork floats.
D. Yes, the marble is less dense than the liquid and sinks while the cork floats.
What is yes, the marble is more dense than the liquid and sinks while the cork floats?
Students observed unknown solid materials. The students conducted three of their four planned investigations to identify the unknown materials.
Investigation Observations
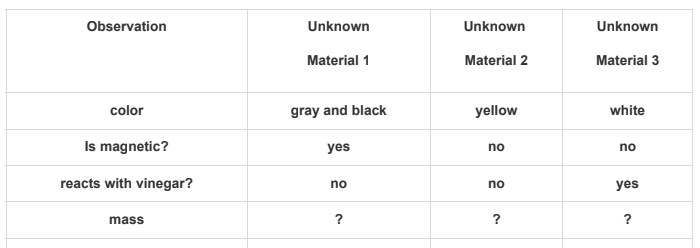
Based on the data collected, which statement best describes one of the unknown materials?
A. Unknown material 2 must be a compound because it is yellow.
B. Unknown material 1 must be a metal because it was attracted to the magnet.
C. Unknown material 2 must have the greatest mass because it is gray and black.
D. Unknow material 3 must be baking soda because it did not react with the vinegar.
What is unknown material 1 must be a metal because it was attracted to the magnet?
What procedure would you perform to test the density of an object?
A. Place the object in water and see if it sinks or floats.
B. Place the object in water and see if it dissolves.
C. Place the object in a citcuit and see if it allows electricity to flow through it.
D. Place the object on a balance and record the amount measured.
What is place the object in water and see if it sinks or floats?
Penny is testing different properties of substances. She places a liquid in a graduated cylinder. She observes the liquid and records the measurement on her paper. What physical property was she testing?
A. length
B. mass
C. density
D. volume
What is volume?
Which object will conduct electricity, conduct heat, and be attracted to a magnet?
A. iron nail
B. glass pan
C. wooden toothpick
D. cotton ball
What is an iron nail?
The table contains information about the densities of objects and what happens when the objects are placed in water.
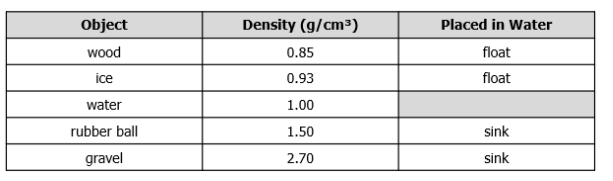
A student has an object with a density of 1.75 g/cm3. Using the data from tyhe table, what will happen when the object is placed in water, and why?
A. The object will float because it is less dense than water.
B. The object will float because it is more dense than water.
C. The object will sink because it is less dense than water.
D. The object will sink because it is more dense than water.
What is the object will sink because it is more dense than water?
John is studying a substance that is a white solid that does not dissolve when mixed with water. What most likely is the substance?
A. salt
B. lemon juice
C. vegetable oil
D. sand
What is sand?
Which of the following substances would be soluble in water?
A. oil
B. syrup
C. sand
D. sugar
What is sugar?
Which property do the following substances have in common: nickel, iron, steel?
A. magnetic
B. mass
C. color
D. volume
What is magnetic?
Which would best explain why a sleeping bag manufacturing company would stuff their sleeping bags with down feathers?
A. The feathers are soft and will not be lumpy.
B. The feathers are good protection from the rain.
C. The feathers are a good thermal insulator.
D. The feathers are easily replaced if they slip out.
What are the feathers are a good thermal insulator?
A student conducts a scientific investigation to determine which items have densities greater than water. The student knows that if an item has a density greater than water, it will sink. The student drops four different items into a beaker of water and records the outcomes in a table.
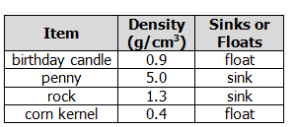
What conclusion can the student make based on the date collected?
A. All of the items have densities greater than water.
B. Water has a greater density than all of the items.
C. The rock and the penny have greater densities than the water.
D. The water is less dense than the birthday candle and the corn kernel.
What is the rock and the penny have greater densities than the water?
Which of the following substances are magnetic?
A. aluminum rod
B. copper wire
C. gold ring
D. silver chain
E. steel
What is steel?
Jan is doing an investigation where he is testing different properties of substances. He decides to put the substance on a balance and record the measurement. What physical property is he testing?
A. Length
B. Color
C. Density
D. Mass
What is mass?
Which property do the following substances have in common: rubber ball, oil, and styriofoam?
A. color
B. state of matter
C. magnetism
D. density
What is density?
A metal pan will heat up quickly when placed over heat. The metal pan is a good thermal ________.
A. resistor
B. insulator
C. conductor
D. accelerator
What is a conductor?
Sam poured oil and water into a jar and mixed the liquids. He let the liquid sit in the jar for 6 hours, and the 2 liquids separated as shown in the diagram.
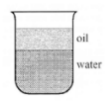
A. The oil is more dense than water and dissolved in the water.
B. The oil is less dense than water and dissolved in the water.
C. The oil is more dense than water and cannot dissolve in water.
D. The oil is less dense than water and cannot dissolve in water.
What is the oil is less dense than water and cannot dissolve in water?
A student is testing different physical properties of substances. He used a laser to shine onto the substance and notices that the laser bounces off of the substance and is shining on the wall. What physical property was the student testing?
A. Magnetism
B. Density
C. Conductivity
D. Reflectivity
What is reflectivity?
Which of the following substances would be a good thermal conductor?
A. wood plank
B. cast iron pan
C. wool blanket
D. styrofoam ice chest
What is a cast iron pan?
Which property do the following substances have in common: sugar, salt, coffee grounds?
A. color
B. solubility
C. magnetism
D. mass
What is solubility?