What is the definition of matter?
Anything that has mass, made up of small particles and takes up space.
What is measurement?
The size, length, or amount of something, as established by measuring
What property of matter do nickel, iron, and steel have in common that other metals do not?
They are magnetic.
The 5 senses can be used to classify this type of physical property:
QUALITATIVE or QUANTITATIVE
Qualitative, because you are able to physically use senses to make observations.
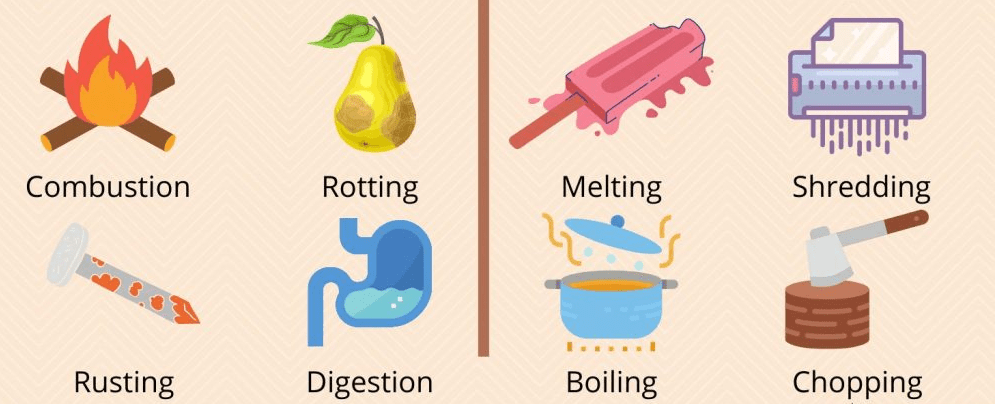
Looking at the images above, which side shows a change in identity or the chemical property of matter. Have reasoning to share.
The images on the left because they show changes occurring that cannot be turned back into it's original form.
Name one object that is matter and one object that is not characterized as matter.
example: Table, door, person, air = all matter!
example: Light or Fire = not matter!
What are properties?
Physical and chemical characteristics of matter used to describe or identify a substance.
Which item(s) from our investigation showed a reaction to all forms of properties?
Which one is NOT a QUANTITATIVE physical property of matter?
melting point, hardness, conductivity, magnetism
Hardness. This is a QUALITATIVE physical property of matter.
Which of the following can cause water to have a chemical reaction?
flammability, rusting, corroding
Rusting- when water on metal dissolves over time it can cause the metal to react by showing rust
True or False: Air is not matter because we can not see it.
False: Air is matter because it is made up of atoms that have a mass and take up space.
What tool measures temperature?
A thermometer.
How hot or cold a substance it is.
Which property are we testing out when we use a heat lamp near certain items?
Thermal Conductor/ Heat
Which one is NOT an example of a QUALITATIVE physical property of matter?
color, odor, taste, texture, density
Density- this is a QUANTITATIVE physical property of matter.
Which of the following is NOT an example of a chemical property?
reactivity, flexibility, corrosion, flammability
Flexibility- this is an example of a physical property
True or False A dog is matter
True a dog has mass and takes up space
What is volume?
The amount of space matter takes up.
When an electric current flows through a metal spoon or a nail what property are we testing out?
Electrical conductivity
TRUE or FALSE...
A physical property can change without the substance becoming a different substance.
TRUE
TRUE or FALSE...
Chemical properties describe the ability of a substance to change its identity.
TRUE
Is an ice cube a solid, liquid, or gas.
Solid
The heaviness of an object. The force of gravity on mass = weight
Which property are we testing out when we use a laser on objects?
Reflectivity
What is a Physical Property?
Physical properties are characteristics of matter that can be observed or measured.
What is a Chemical Property?
Chemical properties are characteristics of matter that describe the chemical makeup of a substance.