Describe particle motion in a gas.
Particles have a lot of energy and move freely.
Which of these are more dense?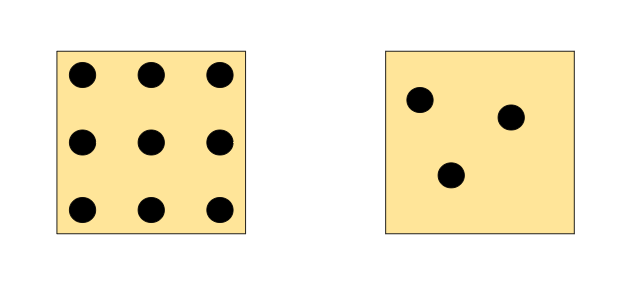
The square on the left.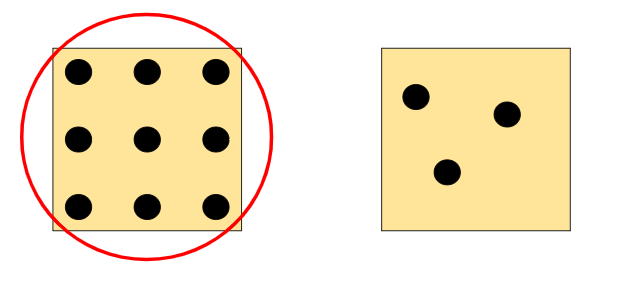
Physical or chemical:
Water freezing
Physical
Balance this equation:
H2 + O2 --> H2O
Daily Double
2H2 + O2 + 2H20
What is a synthesis reaction?
A reaction where two different elements or compounds combine to form a single, more complex substance.
What are the 4 main phases of matter?
Solid, liquid, gas, plasma
Define density in your own words.
How much mass is held in a certain volume of a substance OR how many particles are in a certain amount of a substance.
Physical or chemical: Rust
Explain how you know.
Rust is a chemical change because it is irreversible and changes the composition of the substance from iron to iron oxide.
Balance this equation:
N2 + H2 --> NH3
N2 + 3H2 --> 2NH3
What is a decomposition reaction?
A reaction where one compound splits apart into two or more substances (elements or simpler compounds).
What is an example of a plasma?
Daily Double
Lightning, Fire
Find the mass:
Calculate the mass of a liquid with a density of 2.5 g/mL and a volume of 15 mL
37.5g
Give an example of a chemical property. Explain why it is chemical.
Answers vary.
In your own words, what is the Law of Conservation of Mass?
"Matter is neither created nor destroyed."
What kind of reaction is this?
Zn + HCl -> ZnCl2 + H
Single replacement
Explain in your own words: What are condensation & vaporization?
Give an example of either.
Condensation: Going from a gas to a liquid (Ex: water on the outside of a cold glass)
Vaporization: Going from a liquid to a gas (Ex: evaporation)
Solve the density equation:
A chef fills a 50 mL container with 43.5 g of cooking oil. What is the density of the oil?
0.87g/mL
Explain why density and state of matter can be considered physical properties.
You do not have to change the substance to observe density or state of matter. Also, a phase change does not create a new substance.
Balance this equation:
GaF3 + Cs --> CsF + Ga
Bonus 100pt: What kind of reaction is this?
GaF3 + 3Cs --> 3CsF + Ga
Bonus: Single Replacement
What kind of reaction is this?
CH4 + O2 --> H2O + CO2
Daily Double
Combustion reaction
Explain in your own words: What are sublimation & deposition?
Give an example of either.
Sublimation: Changing from a solid to a gas (Ex: Dry ice)
Deposition: Changing from a gas to a solid (Ex: Frost on a windshield)
Solve the density equation:
A solid magnesium flare has a mass of 1300g and a volume of 743 cm3. What is the density of magnesium?
1.8g/cm3
What are 3 examples of evidence of a chemical change?
1. Gas production
2. Change in odor
3. Unexpected color change
4. Release of energy (light, heat, sound, etc.)
5. Precipitate formation
Balance this equation:
CF4 + Br2 --> CBr4 + F2
CF4 + 2Br2 --> CBr4 + 2F2
What is the difference between an Endothermic and an Exothermic reaction?
Exothermic: Energy is released
Endothermic: Energy is absorbed