Matter Matter
Anything that has mass and takes up space
What is matter?
The measure of the gravitational force of an object
What is weight?
What are the three states of matter?
What is solids, liquids, and gases?
Physical properties of matter can be observed _(with/without)_ changing the identity of the matter.
without
Chemical properties can be observed only when a _______ change might happen.
chemical
A change in color, shape or size is a _______ change.
What is physical?
A metal's ability to attract another substance.
What is magnetic attraction?
Irreversible reactions that involve the creation of one or more new substances
What are chemical changes?
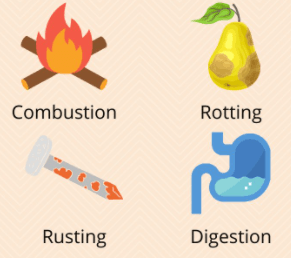
Chemical Changes
Matter is anything that has mass and takes up _____.
What is space?
______________ is the amount of matter in a given space.
What is density?
A state of matter which maintains a fixed volume and shape.
What are solids?
Conductivity, state, malleability, ductility, solubility, and density are all examples of ____________________.
Physical properties
Flammability and reactivity are examples of ___________ properties.
Chemical
Magnetism is a ________ change.
Physical
__________________ is the ability of a substance to burn.
What is flammability?
A change that only alters the form or appearance of the matter; it does not form any new substances
What are physical changes?
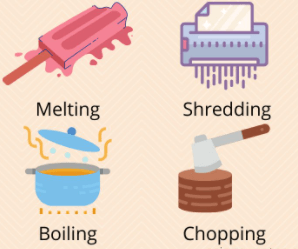
Physical Changes
__________ is the amount of space taken up by an object.
What is volume?
Formula used to determine how dense something is
What is density = mass/volume?
A state of matter which maintains a fixed volume but adapts to the shape of its container.
What are liquids?
Mass and volume are _____ properties because they depend on the size or amount of matter.
physical
_______ means to be able to bend or pound into shapes.
What is malleable/Malleability?
Chopping wood is an example of...
What is a physical change?
The temperature at which liquids turn into gases.
What is the boiling point?
__________ is the amount of matter in an object.
What is mass?
The volume of an irregularly shaped object can be determined by using _____.
What is water displacement?
A state of matter which has no fixed volume or shape, but instead expands to fill and take the shape of its container.
What are gases?
An object that is less dense than water will _______.
float
The new substance as a result of its chemical properties has __________ properties
Different

What is ductile?
A rusting nail is an example of...
What is a chemical change?
The temperature at which solids turn into liquids
What is the melting point?
Name something that is not matter
What is sound or light?
Your ____________ will not change in outer space.
What is mass?
Which state matter is electronically charged gas particles?
What is plasma?
Objects with he same volume do not always have the same _____.
What is mass?
_____________ is the ability of two or more substances to combining to form something new.
What is reactivity?
Change in color or odor, production of heat, fizzing and foaming, and sound or light being given off are all signs that indicate ___________ changes.
Chemical
The measure of how well an electric current can move through a substance.
What is electrical conductivity?
Melting chocolate bar is an example of...
What is a physical change?
State with fastest moving molecules. State with the slowest moving molecules.
What are gases? What are solids?