How many protons are in iron?

26
True or false: Water is non-polar
False: Water IS polar
A molecule with a charge is a ____ molecule (hint: like how a magnet has a charge)
Polar
Many things dissolve in water because water is a ____ ____
Universal solvent
When a solid goes directly to a gas
Sublimation
What is the atomic mass for Lithium?

6.941
In a water molecule ____ has a negative charge
Oxygen
How many oxygen atoms are in 5 water molecules?
5
H2O = 1 O so 5 would be 5
When water freezes it ____
The structure of solids
Lattice
How many electrons are in BOP?
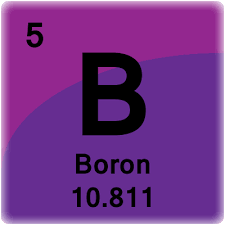


28
This property allows water molecules to stick to other water molecules
Cohesion
Oil does not dissolve in water. When combined, do they make a solution or a suspension?
A suspension
It takes a lot of energy to change the temperature of water. This property is...
High Specific Heat Capacity
Name this molecule and type of bond: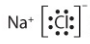
Sodium Chloride
NaCl
Ionic Bond
How many electrons can the 2nd valence shell hold?
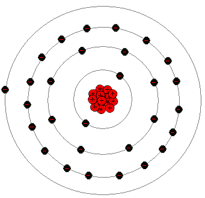
8
This property allows water to stick to other surfaces/things
Adhesion
What type of bond holds a water molecule together?
Covalent
Together
Name this molecule and type of bond:
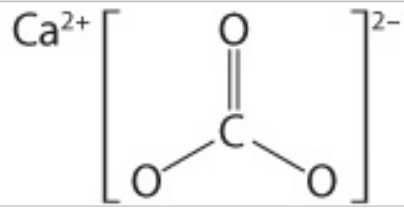
Calcium carbonate
CaCO3
Ionic Bonds
What is the chemical symbol for:
C6H12O6
Surface tension
Capillary action is caused by these 2 properties
Cohesion & Adhesion
This property is why water needs a higher temperature to break the hydrogen bonds and turn water into a gas
High heat capacity
Name this molecule and bond:
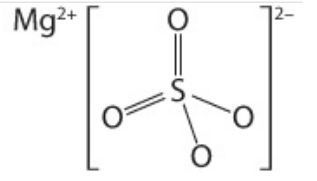
Magnesium sulfate
MgSO4
Ionic Bond