anything that has volume and mass
What is matter?
Atoms from these two elements make up a molecule of water
What are hydrogen and oxygen?
All kinds of matter are made up of tiny ______.
What are particles or atoms?
The chemical formula for this model:
H-H-S-O-O-O-O
What is H2SO4?
The following are all subatomic particles EXCEPT _____:
protons, neurons, electrons, neutrons
What are neurons?
the smallest particle of an element
What is an atom?
The number of different elements in ZrSiO4.
What is 3?
The number of atoms a single molecule can contain.
What is 2 or more?
The chemical formula for this model;
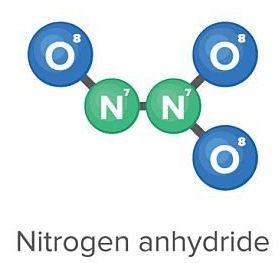
What is N2O3?
This element has the symbol Na.
What is sodium?
a substance that is made entirely from one type of atom
What is an element?
a number written below and to the right of a chemical symbol that shows the number of a specific type of atom present
What is subscript?
The reason why graphite is softer than diamond.
What is the different arrangement of atoms?
The missing part of this model of a water molecule.
What are lines connecting the atoms?
This is the sport that Mr. Floyd plays most often.
What is table tennis?
the simplest unit of a chemical compound that can exist, formed when two or more atoms join together chemically
What is a molecule?
The chemical formula shows the number and type of atoms in one __________ of a substance.
What is a molecule?
These are the sections of crystals that are repeated in patterns to build their structure.
What are subunits?
The three items shown in this model:
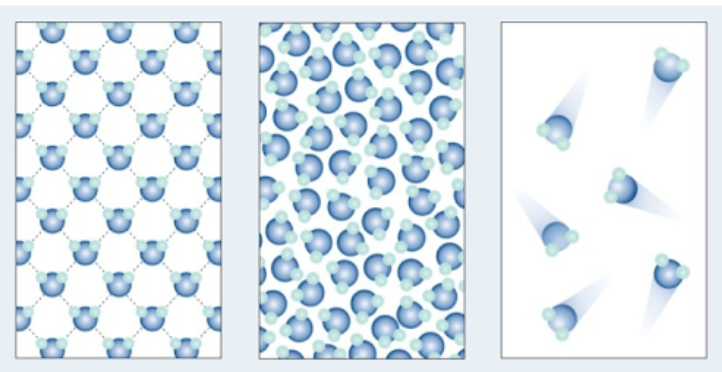
What are the states of matter (solid, liquid, gas)?
These are the names of the 7th grade math teachers.
What are Mrs. McSweeney and Mr. Cooke?
a solid material whose atoms, molecules, or ions are arranged in an ordered pattern
What is a crystal?
The total number of atoms in a molecule of C6H12O6.
What is 24?
These subatomic particles have a positive charge.
What are protons?
The type of solid structure shown in this model:
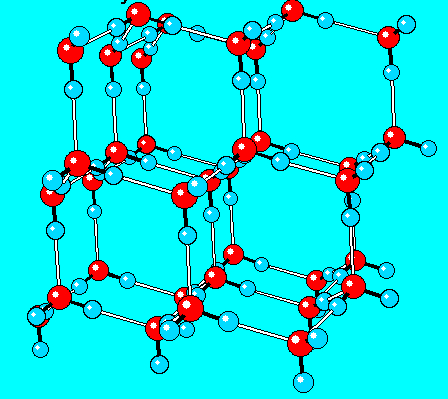
What is a crystal?
This is the number of protons in an atom.
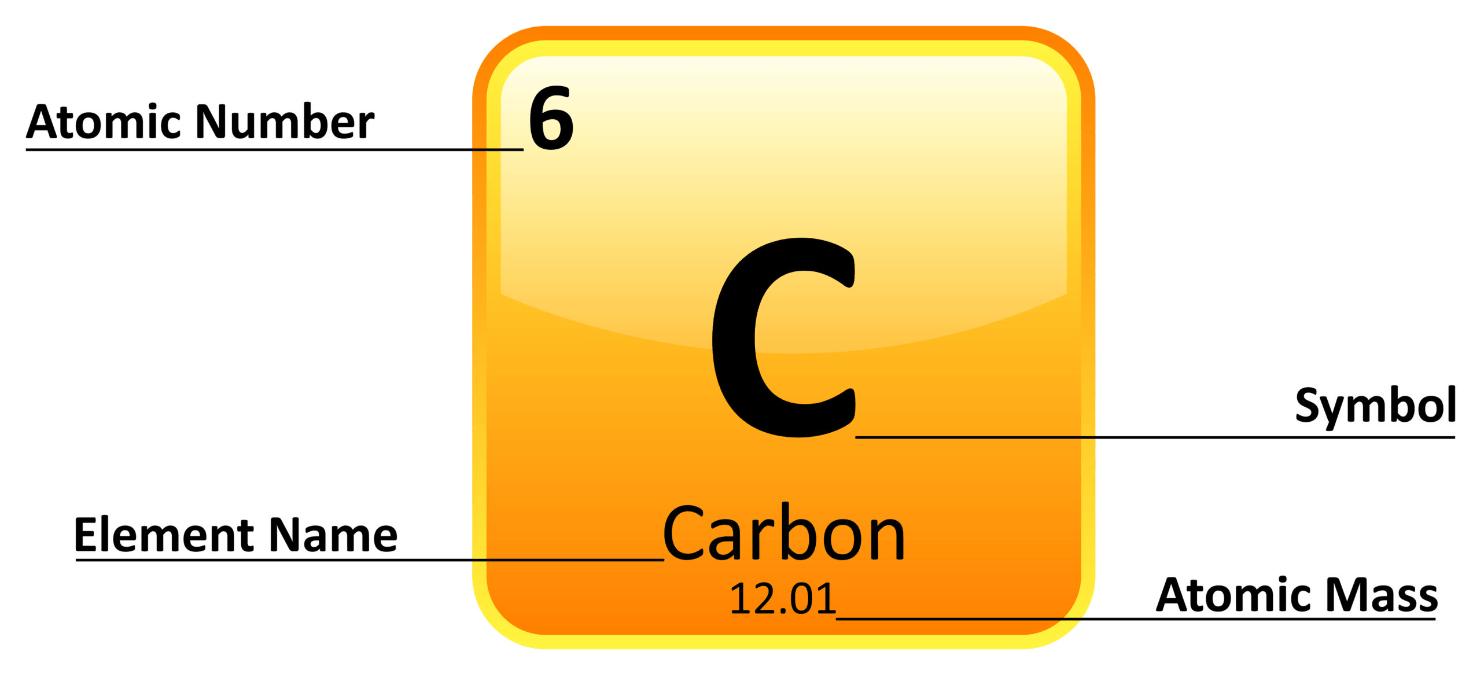
What is atomic number?