Calculate the molar mass of Na2SO4 to 5 sig figs
142.05 g/mol
Looking at the three stars on the graph, which colored star represents each of the following solutions?
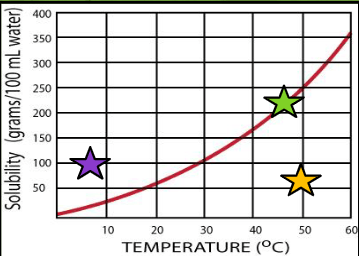
Purple: Supersaturated
Green: Saturated
Yellow: Unsaturated
Which substance below would turn blue litmus paper red, taste sour, and react with metals?
Substance A: pOH = 3
Substance B: pOH = 11
Substance B because it has a pOH of 11 and therefore a pH of 3
What is the boiling point of this substance?
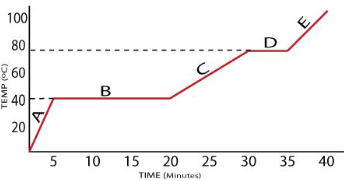
80oC
Which gas law variables have an inverse relationship?
Volume and Pressure
How many moles of H2 are produced from 5.8 moles of NH3?
2NH3 → N2+ 3H2
8.7 moles H2
How much more solute can be dissolved in 100 mL if the temperature is increased from water at 30oC 40oC?
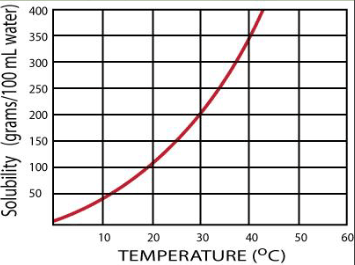
150g more
If a solution’s concentration is [H+] = 1.0 x 10-2 M, what is its pOH?
12
Which letter represents the enthalpy of this reaction?
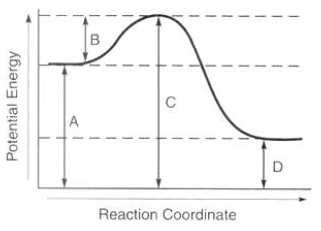
A
A gas occupies 900.0 mL at a temperature of 27°C. What is the volume at 132.0°C?
1200 mL or 1.2 L
What is the percent yield of a reaction if 15.0 g of CaO were actually produced when 16.8 g of CaO were predicted to be made?
89.3%
What is the molarity of 139 g of HCl dissolved in 2.5 Liters?
1.5 M
Identify the conjugate acid & conjugate base in this reaction:
H2CO3 + H2O → HCO3- + H3O+
Which theory discusses conjugate pairs?
Conjugate acid = H3O+
Conjugate base = HCO3-
Bronsted-Lowry
Write ENDO/EXO by each of the following:
Reaction absorbs heat
Condensation
ΔH is negative
Freezing
Heat is a product
1. ENDO
2. EXO
3. EXO
4. EXO
5. EXO
A 5.00 L container holds an unknown gas at 2.50 atm and 310 K. How many moles of gas are present?
0.490 moles
How many grams of water are needed to produce 230. grams of CO2?

188g H2O
A student dissolves 15 grams of salt in 250 mL of water. What is the percent by mass of this solution?
5.7%
What would be the name and formula of acids (a) and (b) below?
(a) H2SO4 (b) Hydroselenic acid
(a) Sulfuric acid
(b) H2Se
Calculate the amount of heat needed to raise the temperature of 50.0 grams of water from 4.0oC to 36oC
6700 J
What pressure is required to compress 196.0 L of air at 1.00 atmosphere into a cylinder whose volume is 26.0 L?
7.54 atm
How many atoms of Cu are in 385 grams?
3.65 x 1024 atoms
If each dot represents one mole of solute, what are the concentrations of Solutions D-F below?
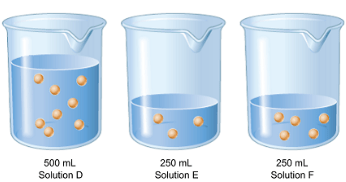
Solution D: 16 M
Solution E: 12 M
Solution F: 20 M
Identify parts A-D of the neutralization reaction below:
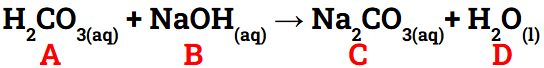
A. Acid
B. Base
C. Salt
D. Water
Place the following substances in order from smallest temperature change (1) to largest temperature change (5)?
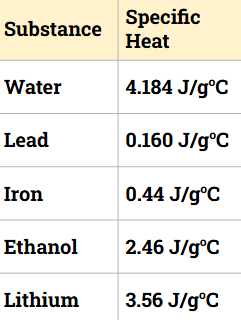
1) Water
2) Lithium
3) Ethanol
4) Iron
5) Lead
How does increasing the temperature on an endothermic reaction shift the chemical equilibrium?
the reaction would shift right to favor the formation of more products
Identify the limiting and excess reactant from the observations in the table below.
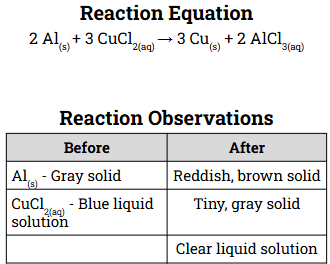
CuCl2 must be the limiting reactant as there is no blue liquid remaining after the reaction but there is gray solid (Al) remaining after.
Which solution most likely contains glucose C6H12O6 and how did you know?
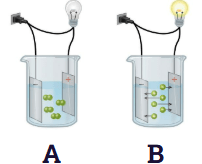
Solution A because it does not form ions nor conduct electricity.
Which diagram (LEFT or RIGHT) represents a weak acid and how did you know?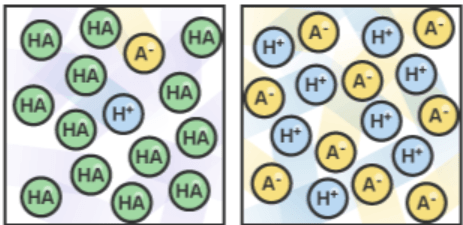
Left because weak acids do not dissociate 100%/only partially dissociate in solution
Given the reaction and the standard enthalpy changes below, calculate the ΔH of the reaction:
4 NH3(g) + 5 O2(g) → 4 NO(g) + 6 H2O(g)
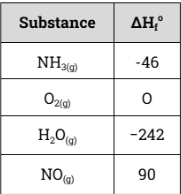
-908 kJ
Put a check mark by the stressors that would increase NH3(g) production:
N2(g) + 3 H2(g) → 2NH3(g) + heat
Increase H2(g) concentration
Decrease Pressure
Increase Temperature
Decrease Volume
Increase NH3(g) concentration
✅ Increase H2(g) concentration
✅ Decrease Volume