Type of reaction:
3 A + 1 BC3 --> 3 AC + 1 B
What is a single replacement reaction?

1 mol of C
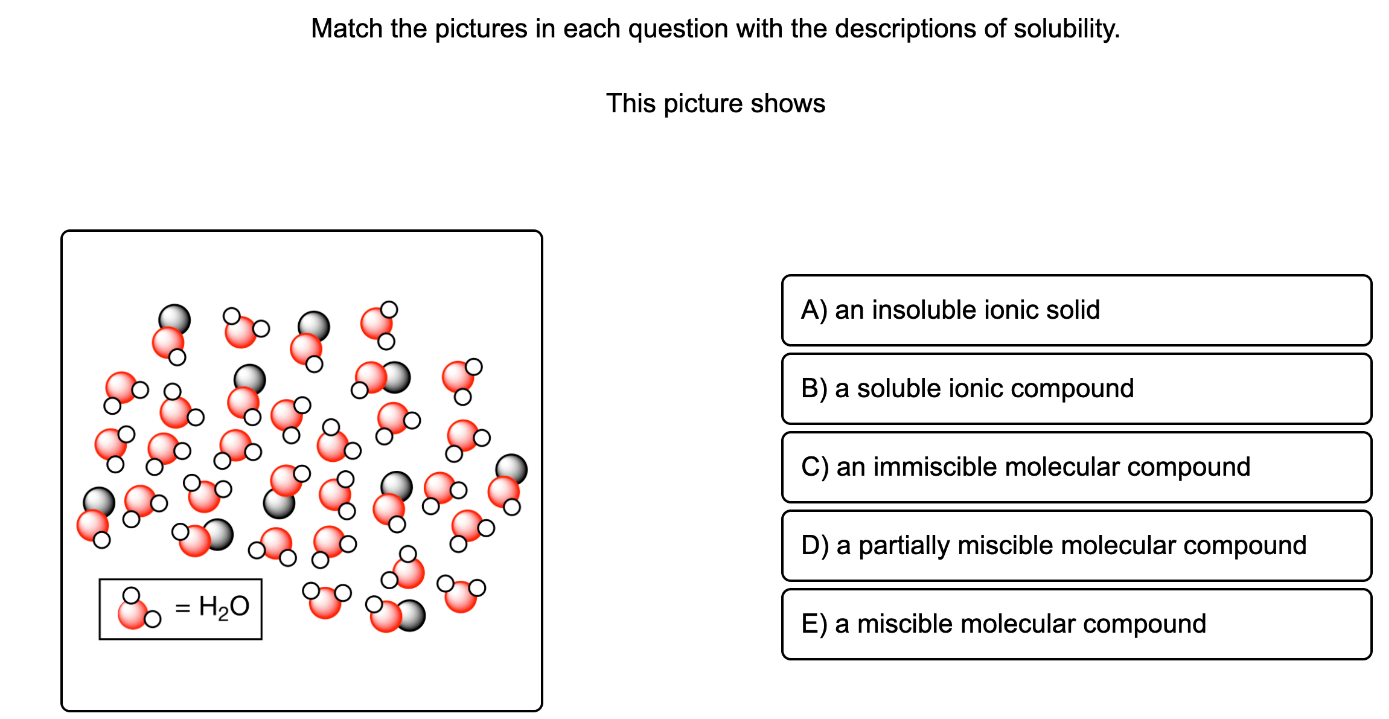
This picture shows a miscible molecular compound. The solute molecules are dispersed throughout the solution.
The predominant (strong) intermolecular force between molecules of I₂ is ?
Move over cheesesteak, Philadelphians consume 12 times as many of these compared to the average American.
What is the pretzel?
The chemical formula(s) for the product(s) of the following reaction.
Al + O2 -->
What is Al2O3?
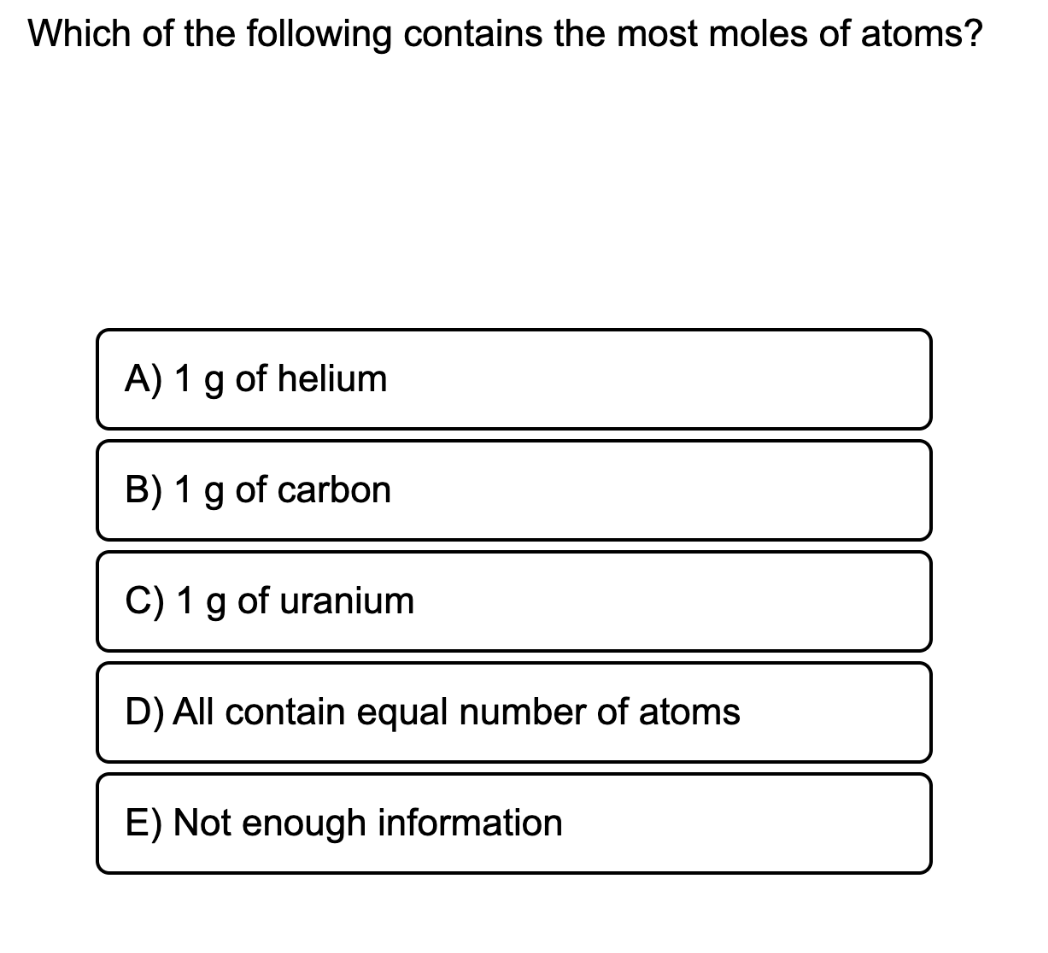
1 gram of helium
(To convert from grams to moles, you need to divide by the molar mass. Since each of these options has the same mass, dividing by the smallest molar mass will yield the most moles of atoms. Since helium has a lower molar mass (4 g/mol) than carbon (12 g/mol) or uranium (238 g/mol), it must contain the most moles.)
What are the ions in an aqueous solution of barium chlorate - Ba(ClO3)2?
What are Ba2+ and 2 ClO31-?
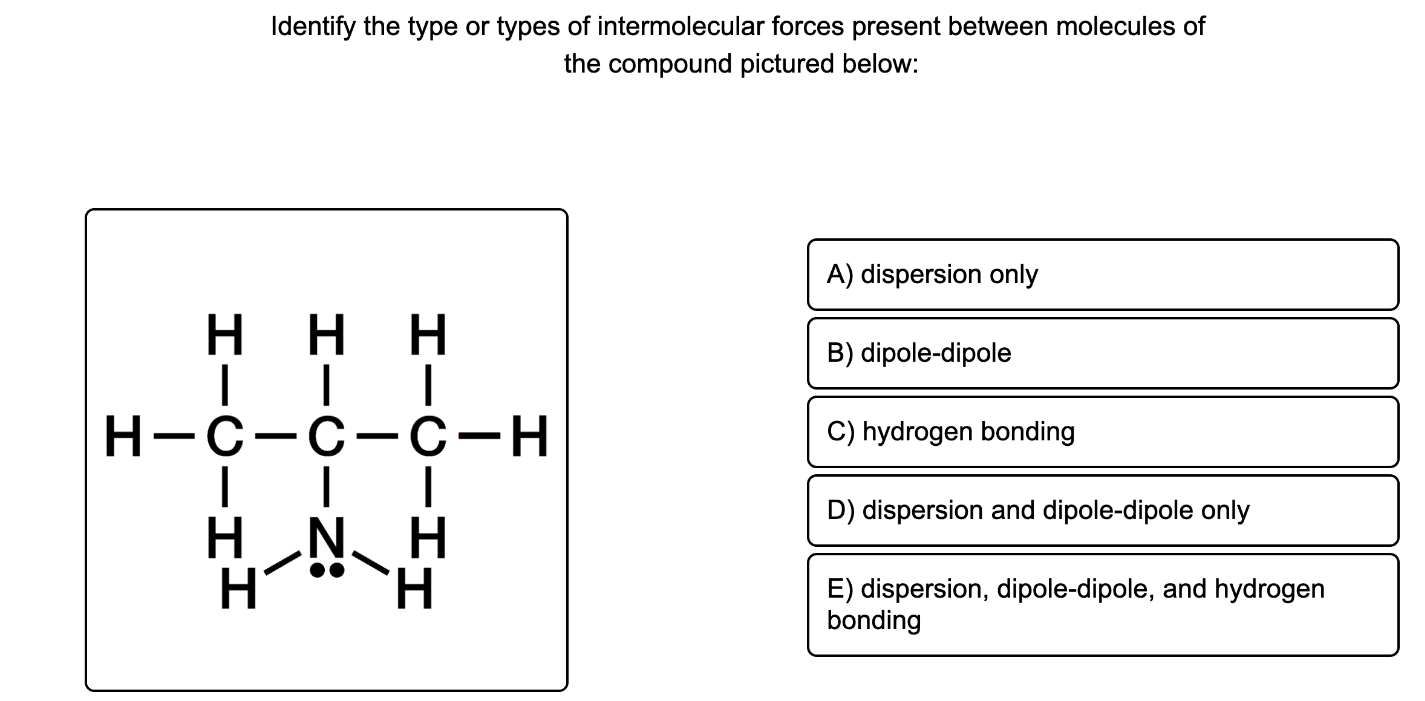
This molecule is polar, and it has a hydrogen covalently bonded to a nitrogen. Therefore, London dispersion forces, dipole-dipole forces, and hydrogen bonding are all present.
However the strong force present is hydrogen bonding.
Name this company from its logo:

What is Huawei?
The chemical formula(s) for the product(s) of the following reaction.
Co + Mg(NO3)2 -->
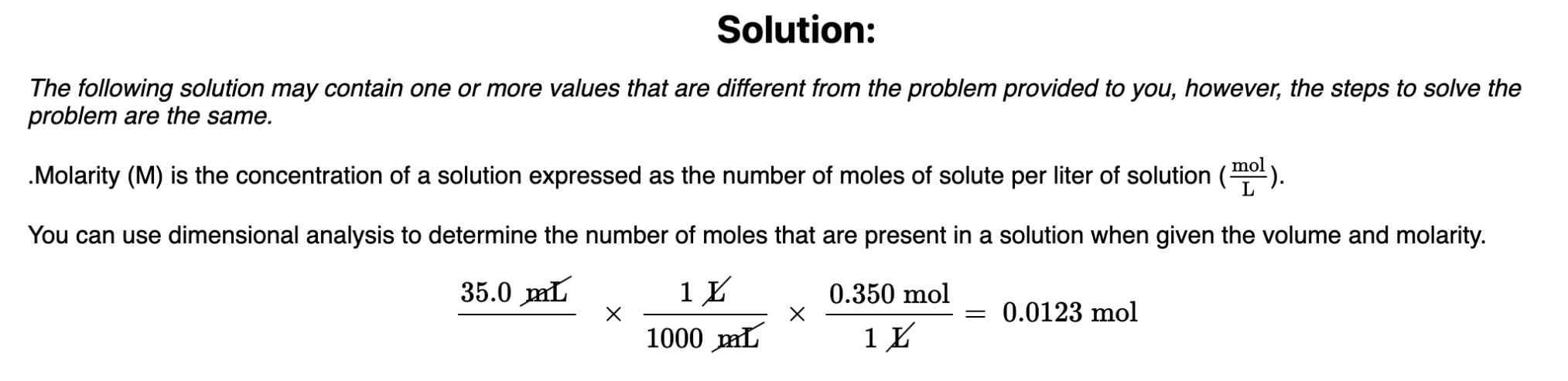
How many moles of HCl are there in 35.0 mL of 0.350M HCl?
0.0123 mol
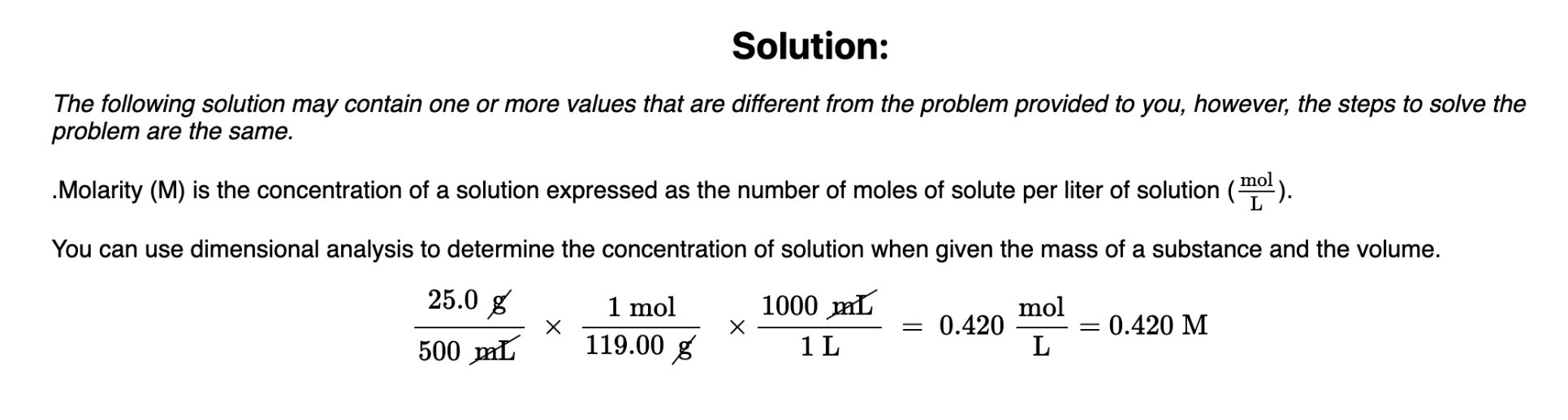
If 25.0 g of KBr (MM = 119.00 g/mol) are added to a 500.0 mL volumetric flask, and water is added to fill the flask, what is the concentration of KBr in the resulting solution?
0.420 M
An unknown solid has the following properties:
High melting point
Soluble in water
Brittle (not malleable)
Does not conduct electricity as a solid but does when dissolved in water
What type of solid might this be? Describe how each of the properties listed above arise from the internal structure of this type of material.
This is most likely an ionic solid.
The high melting point: Ionic bonds are very strong and hard to break due to the full (not partial) positive and negative charges of the particles involved.
Soluble in water because the charged ions are good at being surrounded and pulled away by polar water molecules. "Like dissolves like"
Brittle because the lattice structure of an ionic solid is very regular and repeating with each anion surrounded by cations and vice versa. When part of the lattice is deformed (struck by a hammer, for example) and cations end up next to cations and anions end up next to anions, the lattice will break apart.
To conduct electricity there must be mobile, charged particles. In an ionic lattice, there are charged particles but they are locked in a rigid structure and can’t move. However, when the ions are separated and dispersed throughout water, the charged particles are now mobile and can allow for the flow of electricity.
Name this famous person by their recent browser history:
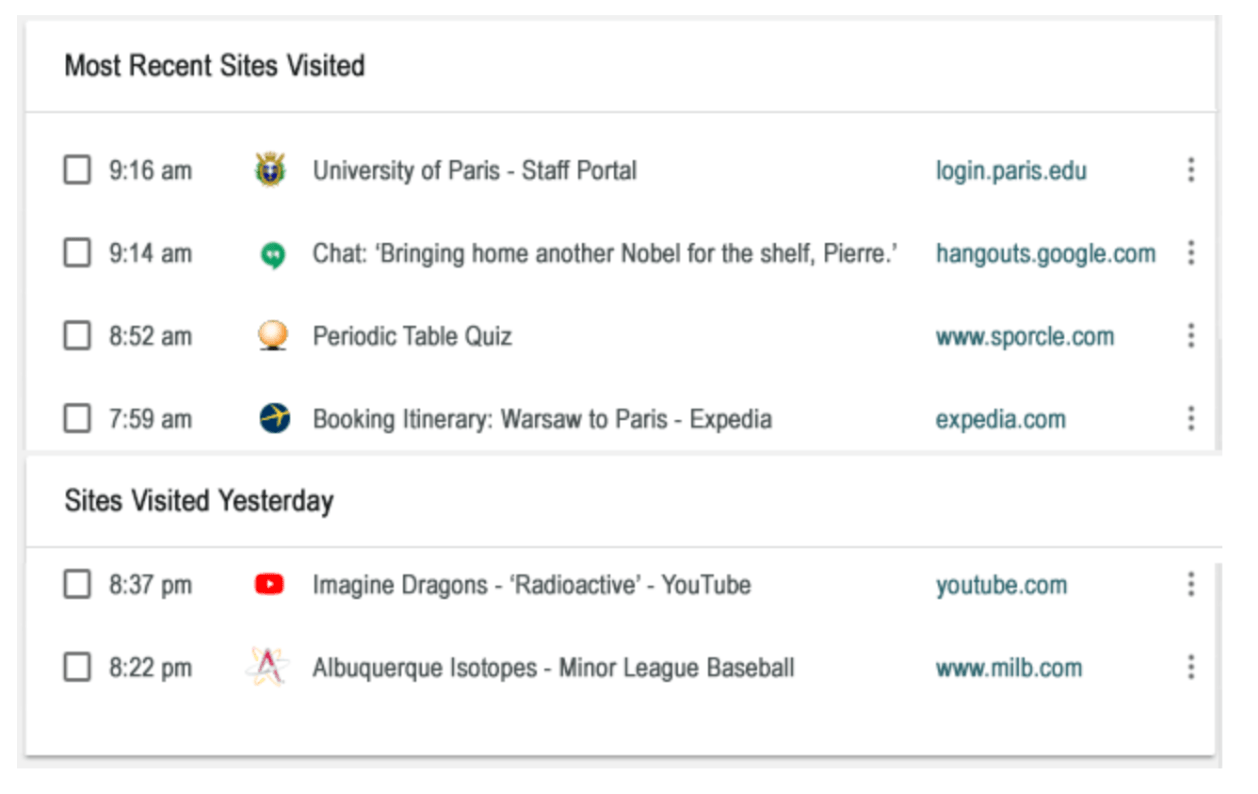
Who is Marie Curie?
The chemical name of the precipitate formed in the reaction of Pb(ClO3)2 and (NH4)2SO3?
What is Lead (II) sulfite?
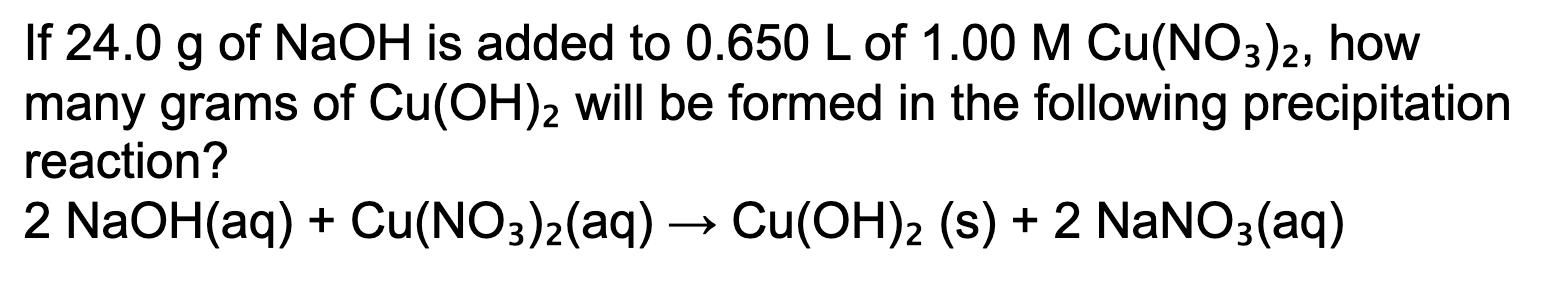
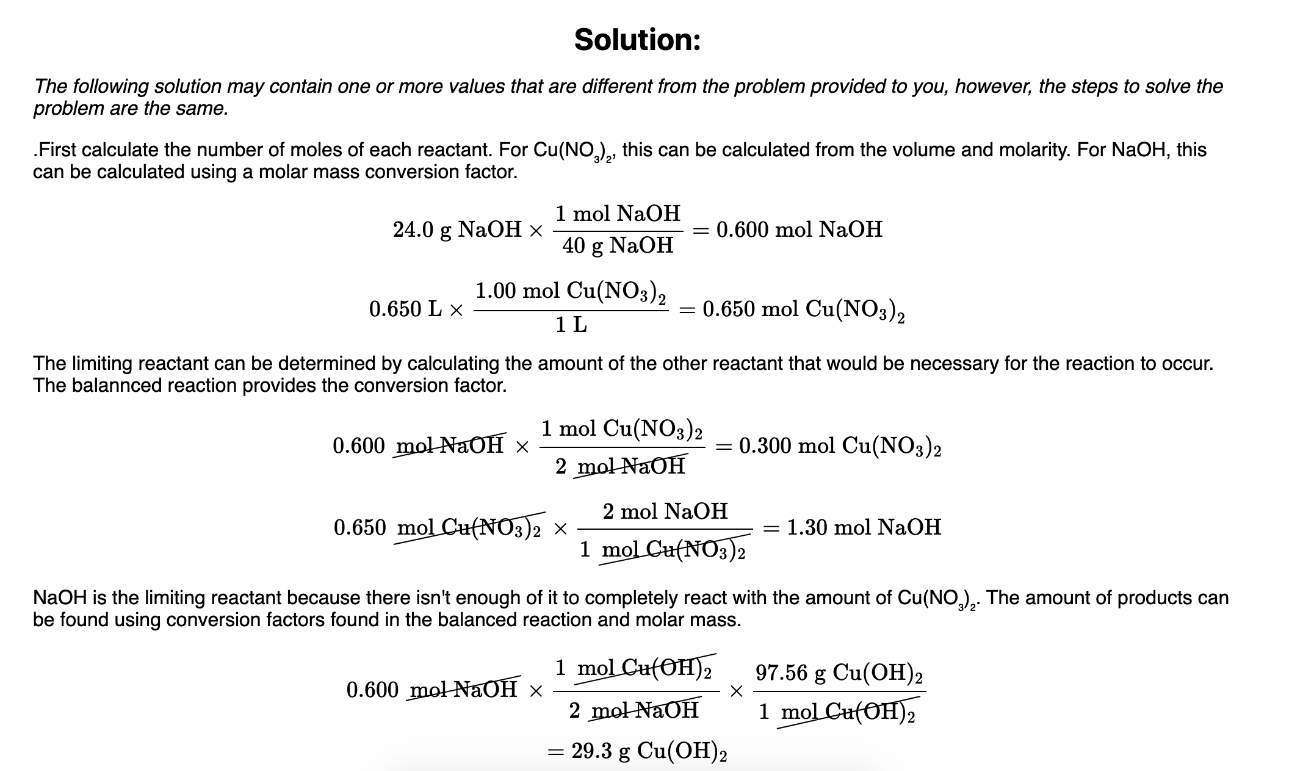
29.3 grams
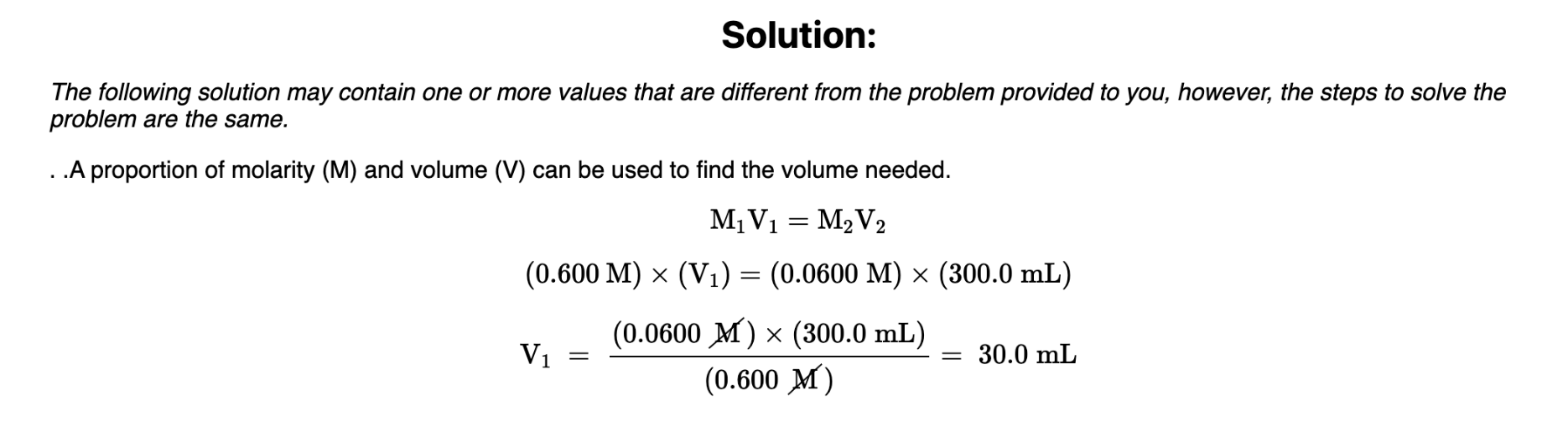
How many mL of 0.600 M LiCl would be required to make a 0.0600 M solution of LiCl when diluted to 300.0 mL with water?
30.0 mL
An unknown substance is found to have a relatively low melting point and does not conduct electricity as a solid or a liquid. The solid does not dissolve in water but is soluble in hexane (C6H14). What is the force of attraction that holds this solid together?
What is London dispersion force?
low melting point (likely molecular)
Not electrically conductive as a solid (can't be a metal)
Not electrically conductive as a liquid (can't be a ionic)
Not soluble in water but is in hexane (non-polar so no D/D and no HB)
Name this 80s movie from hits hit song.
What is the breakfast club?
The spectator ions in the following reaction:
Fe(NO3)3 + Ba(OH)2 -->
What are Ba2+ and NO31- ?

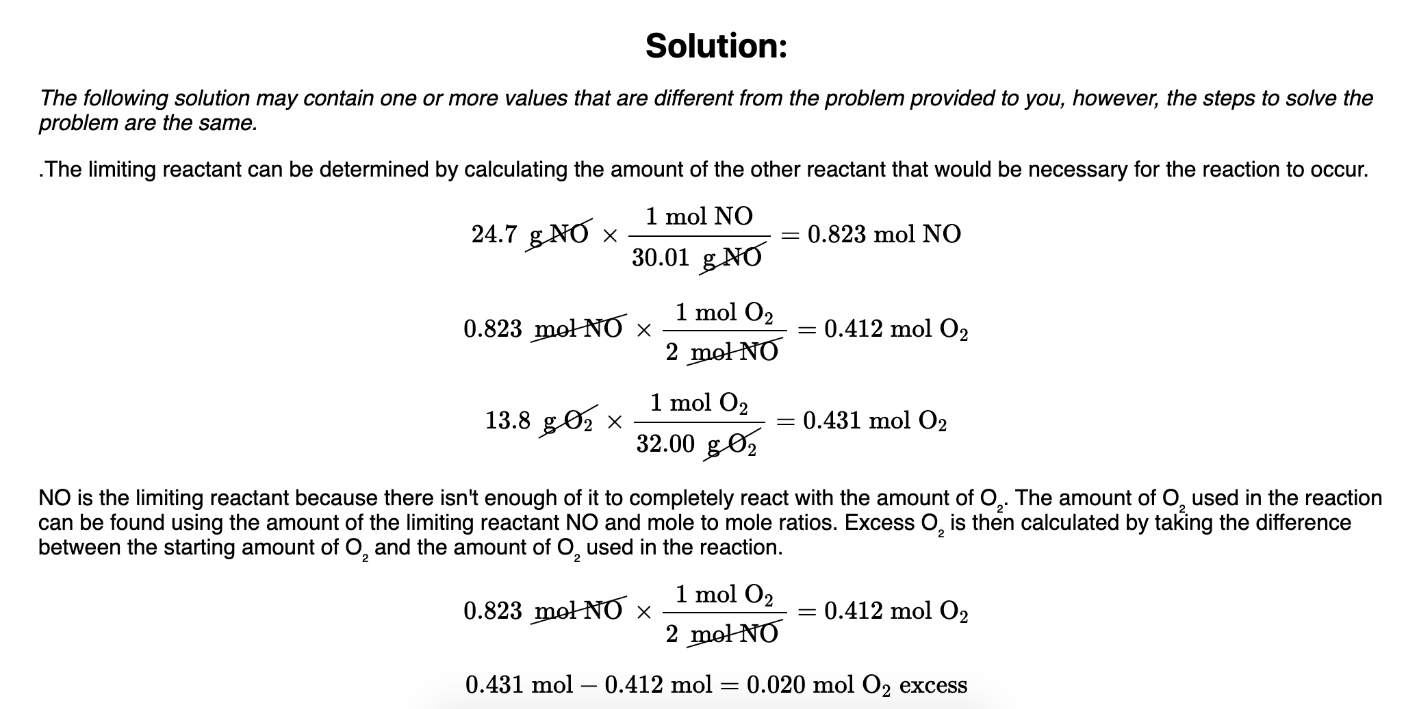
0.020 mol of O2 excess (leftover)
A 250.0 mL solution is properly prepared using 20.0 grams of MgCl2 (95.2 g/mol). However the student forgets to cover the solution with Parafilm and some of the water in the solution evaporates overnight. If the new volume of the solution is 200.0 mL, what is the molarity of the solution?
What is 1.05 M?
Place the following substances in order of increasing boiling point (lowest to highest): H2, NH3, Li2O, N2
(lowest) H2, N2, NH3, Li2O (highest)
Name the movie from 2014 based on this quote:
Interstellar