What is the chemical symbol for Mercury?
Hg
What are valence electrons?
The electrons in the outermost shell of an atom.
What do the dots in lewis dot structures represent?
Valence electrons
What is the molar volume of any gas at STP?
22.4 L/ mol
What is the difference between atomic number and atomic mass?
Atomic number is the number of protons. Atomic mass is how much the atom weighs (protons + neutrons)
All elements in group 14 have 4 valence electrons.
Draw the dot structure for sulfur.

2 K (Potasium)
1 C (Carbon)
2 O (Oxygen)
What is the molar mass of the following element:

95.95 g/mol
Which element has the LOWEST electronegativity?
I, Br, Cl, F
Iodine (I)
What types of ion will Tl form?
cation
What is the molar mass of NaClO?
74.44 g/ mol
Which types of elements make up most of the periodic table?
metals
Rank the elements by increasing ionization energy:
Ca, V, Ti, Sc
Ca, Sc, Ti, V
Draw the bond between Sodium (Na) and Chlorine (Cl).
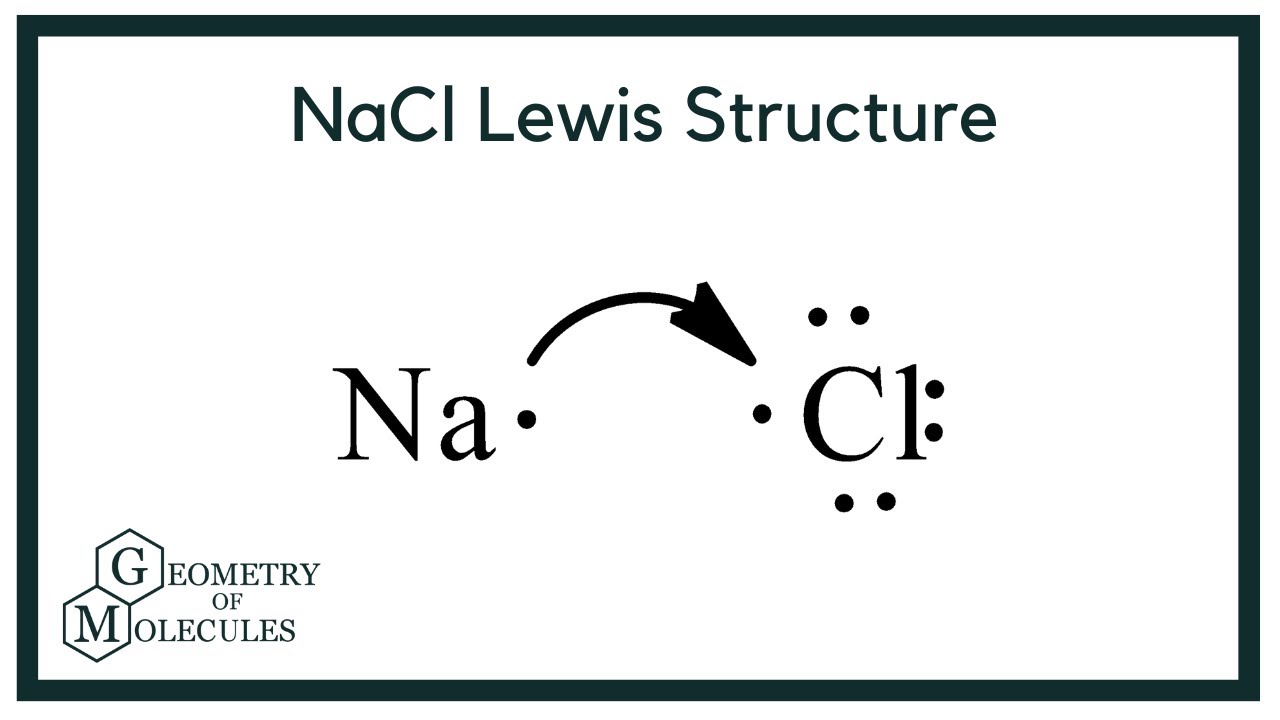
what conversion factor will we need in order to go convert from particles to moles?
avogadro's number
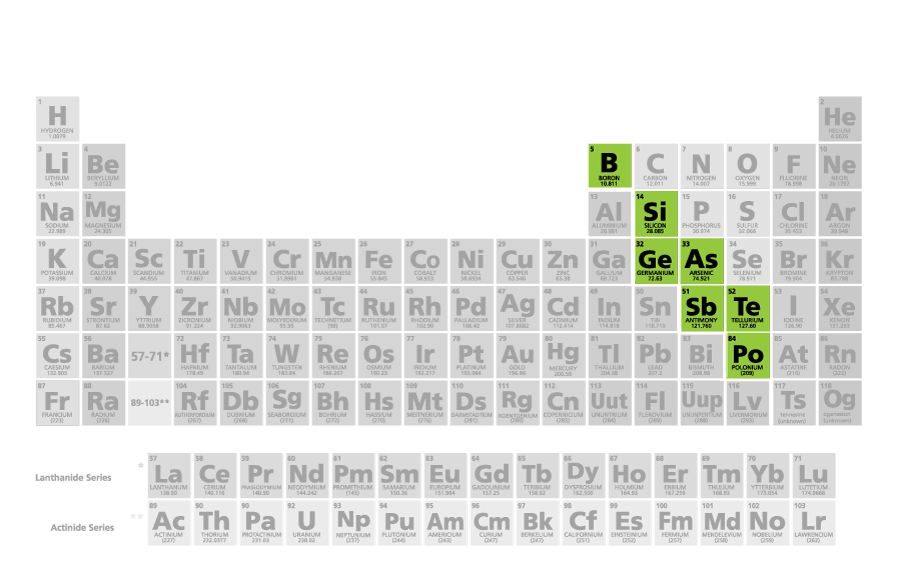
Which type of elements are shown below:
Explain why ionization energy decreases as you go down a group.
Elements down a group have more electron shells shielding, so the attraction between valance electrons and protons is a lot weaker.
What would the formula be for the following bond:

MgCl2
How many grams are in 3.35 moles of NaOH?
39.997 grams