What do the symbols q,m,c, and 𝚫T stand for?
q= heat energy
m= mass
c= specific heat
𝚫T= change in temperature
What is the general definition of enthalpy?
The heat content of a substance.
What is the definition of equilibrium
Is a state of a reaction in which both reactants and products are present in concentrations.
PbI2=5.30 x 10-3 M
What is the Ksp
Ksp=5.96 x 10-7
Particles must have what 3 things in order to create an effective/successful collision.
Particles must collide, they must have sufficient energy, and they must have the correct orientation.
How do you find the enthalpy of a reaction if you given the substance and heat of the solution?
qsol=-qrxn
enthalpy=qrxn/mol
If one mole of CO2 is cooled what determine the sign of 𝚫S
-𝚫S
What is the simple equilibrium expression equation?
( Using A,B,C,D)
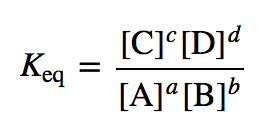
What are the 4 stressors on a system at equilibrium?
Concentration, temperature, pressure, and volume.
What are the five factors affecting reaction rates?
Nature of the reactants, concentration, surface area, temperature, and catalysts.
How do you find 𝚫Hf of a reaction
𝚫Hf=products-reactants
What is Gibbs free energy general definition?
The energy that is available to do work.
If Keq is larger than 1 what side does the equation favor?
The products.
If the pressure in a system goes up it will shift to the side with the ____ gas moles.
Least
Rate laws are used to determine the effect each ______ has on the rate
Concentration
If the reactants side equals 2863 and the product side equals 1927 what does the 𝚫Hbonds equal
𝚫Hbonds=936KJ
How do you know if a reaction spontaneous or not
A spontaneous reaction needs -𝚫G and +𝚫S
What is the normal solubility constant expression?
( Using A and B)
Ksp=[A+]a[B-]b
If decrease the volume in a system it will shift to the side with the ____ gas moles.
Least
Calculate the rate constant
https://docs.google.com/document/d/1AcKGl2l9fHrOxhu6i5bXY7EQxjvJseh6BvYVUB3qR3g/edit
(Copy and paste link)
K= 0.02
If a substance goes from a gas to a solid what phase change is that
Deposition
If delta H is higher than 0 is the reaction endothermic or exothermic?
Is this graph endothermic or exothermic?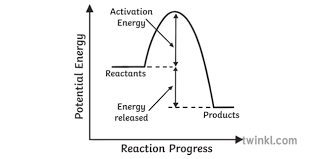
Endothermic
Exothermic
The smaller the Ksp value the _________ a substance is able to dissolve.
Less likely
If heat is on the products side and heat is added which side will the reaction shift to?
The left.
Find the rate order for each substance and find the overall rate order
https://docs.google.com/document/d/1SN7YYilUY13GPNH4tws6oKoV04jR5DFGpMEs1_oHpZY/edit
(Copy and paste link)
A= 1
B= 2
Overall Rate Order= 3