What is -1
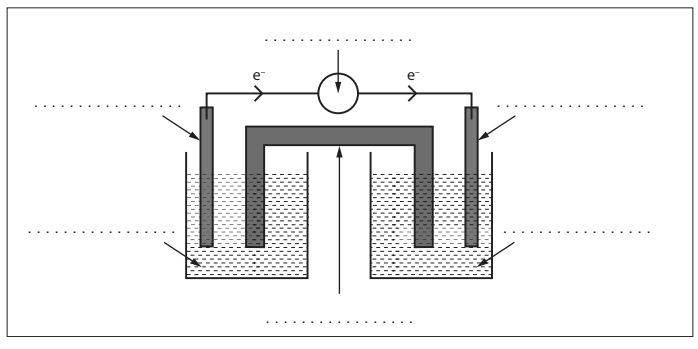
Identify the anode and the flow of electrons

What is zinc electrode? From right to left
Increasing the amount of Hydrogen would move the equilbirum to the
Name three renewable energy sources?
What is wind, solar, biomass, geothermal and hydro
How does a catalsyt affect equilibrium
What is it doesn't! It only allows equilibrium to be established faster.
What is not a definition of oxidation?
Gain of oxygen
Loss of hydrogen
Loss of electrons
Decrease in oxidation number (state)
What is decrease in oxidation number?
What is the function of the salt bridge?
To maintain the balance in the half cells by allowing charged ions (K+ or NO3-) to move from the salt bridge into the half cells.
DECREASING the amount of water would
Shift the equilibrium to the right
Name THREE fossil fuels
What is coal, oil and (natural) gas
What is the name of splitting uranium atoms
What is fission
What is the oxidation state of oxygen? How does the oxidation state of oxygen change in hydrogen peroxide (H2O2)
What is -2 and (-1) in hydrogen peroxide?
In the following voltaic cell which electrode would lose mass
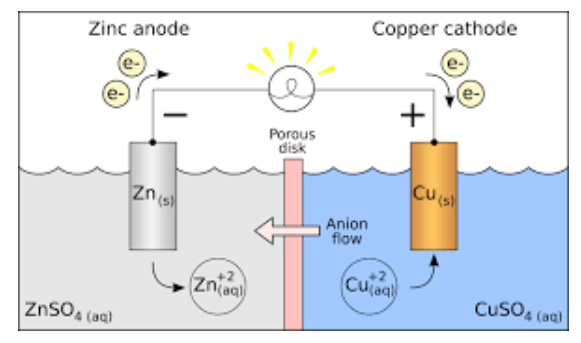
What is zinc
According to the equation what would happen if we increased pressure

Shift to the right (less number of moles)
Describe the greenhouse effect
What is gases (CO2, H2O and CH4) thickening the atmosphere trapping in more infrared light (heat)
What is a good way to get rid of waste from a nuclear power plant
What is bury it deep in a concrete vault with a thick lead lining
Balance the equation and indentify the species undergoing oxidation?

What is 4, 3, 2 and iron
Write an equation for the following voltaic cell

What is
This is an exothermic reaction. At high temperature which reaction would be favoured 
What is the reverse
Name and write the formulas for 3 greenhouse gases
What is water, methane and carbon dioxide
Determine the half life of this substance
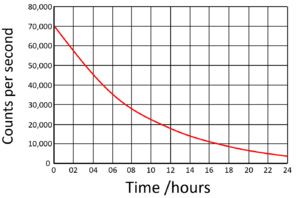
What is 6 hours
Show with working which species is undergoing reduction?

What is iodine?
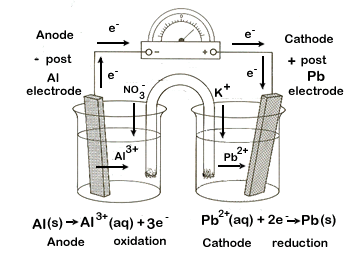
Label the following in this voltaic cell between aluminium and lead
What is
Explain favourable conditions to produce a high amount of nitrogen dioxide (the reaction is exothermic)

High pressure, low temperature and adding more reactant.
Identify a renewable source of energy. Describe the benefits and drawbacks of using this energy source.
Answers will vary
What is a benefit and disadvantage of using radioisotopes in medicine? Should short or long half life substances be used.
Benefit: Can be used to diagnose tumors and treat cancer. Long half life substances can make you very sick.
Disadvantage: Exposes hospital staff and equipment to radioactivity. Only short half life substances should be used.
