-ΔH
What is Exothermic?
What is the formula for ΔH⦵f?
What is Sum of Products- Sum of Reactants (opposite)
What is the functional group COOH called
What is a carboxyl group?
True or False. Alkanes will react spontaneously with bromine.
The IHD of Benzene
What is Four?
 Endothermic or Exothermic?
Endothermic or Exothermic?
What is endothermic
What is the average bond enthalpy equation
Which compounds are members of the same homologous series?
A. propanal, propanone, propanoic acid
B. propane, propene, propyne
C. hexan-1-ol, hexan-2-ol, hexan-3-ol
D. ethanol, propan-1-ol, butan-1-ol
What is D
Hex-1-ene will react with bromine to form......
The color will change from brown to ..........
What is 1,2 dibromohexane and colorless
How many hydrogen environments will this have 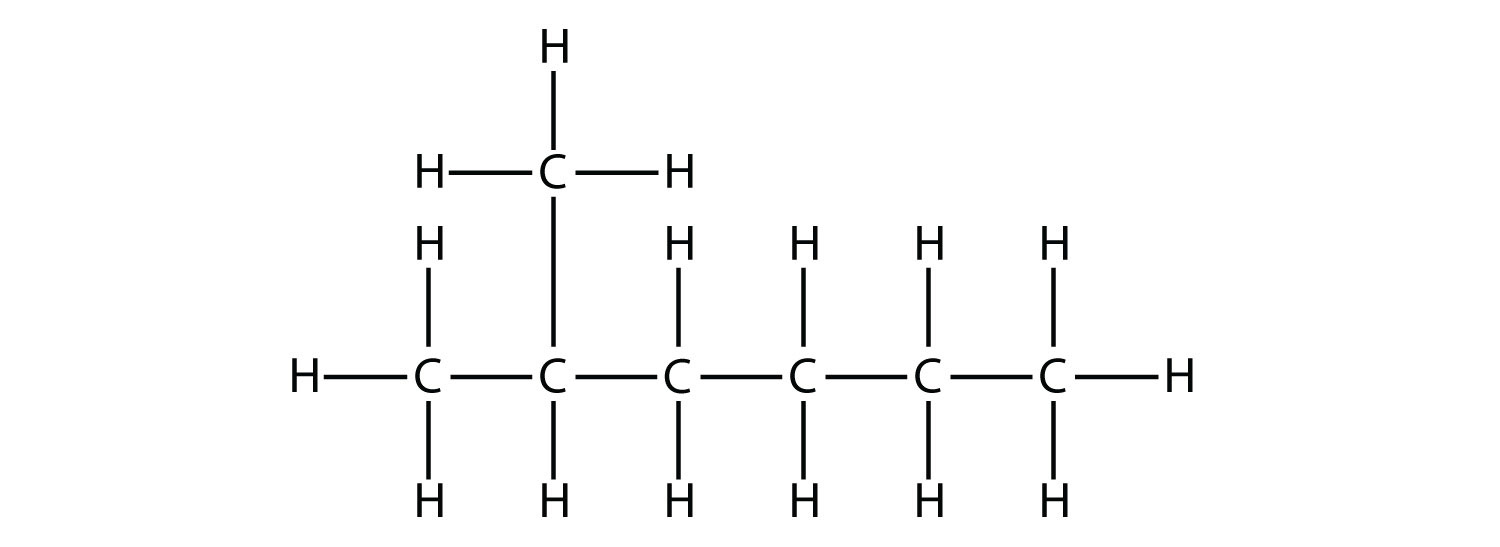
What is 3
Has high energy reactants but low energy products
What is exothermic?
Which molecule has the highest average bond energy?
Chlorine
Oxygen
Nitrogen
What is Nitrogen (triple bond)?
What is the general formula for an alkane?
What is Cn H2n+2
Draw the polymer formed when ethene reactants?
What is polyethene (-CH2-CH2)
The IHD of Serine

What is 1
Q= mCΔT.
What will be the units for Q and C?
What is Q= Joules (J)
C = j/g/degrees C or K
Calculate the average bond energy using
CH4 (g) + Cl2 (g) → CH3Cl (g) + HCl (g)
Section 11 of the data booklet.
What is –99 kJ/mol
Which would have the LOWEST boiling point?
a) Ethane
b) Ethene
c) Ethanol
d) Ethyene
D) Ethyne. It is non polar and has the lowest mass.
The conditions needed to convert a primary alcohol to a carboxylic acid
How many peaks will ethane have on a 1HNMR Result
What is 1? (only one hydogren environment)
In an endothermic reaction heat will be transferred....
What is from the surroundings to the system
Which chemical would have the highest average bond enthalpy
Propanol
Propane
Propanone
Propanoic Acid
What is Propanoic Acid?
Which would have the highest boiling point?
Propane
Propan-1-ol
Propanone
Propanal
What is Propan-1-ol (only chemical that has hydrogen bonds)
What are the expected products of a reaction between pent-2-ene and hydrogen bromide?
What type of reaction is this
What is 2-bromopentane and hydrogen (H2)
Electrophilic Addition
What is the IHD of Ibuprofen
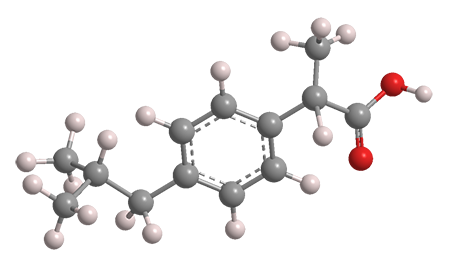
What is 5