The amount of protons in Germanium
What is 32 Protons
Protons + Neutrons = ____________
Protons + Neutrons = the mass number of an atom
The atomic mass of Thorium
What is 232.04 G
How many electrons does Sulfur-34 have?
16
How Do you solve for average atomic mass?
1. Move the decimal over two spots to the left for perfent abundance.
2. Multiply the decimal by the mass of atom (amu)
3. Add the numbers you get from each set.
The number of Protons in a Copper-65 atom
What is 29 protons?
How many neutrons does Magnesium-26 have?
14
Calculate the average atomic mass of Chlorine
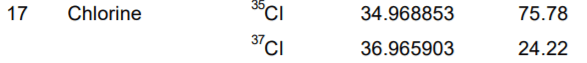
What is 35.45 amu?
Number of electrons=
Number of protons
Calcium-43 has how many neutrons?
What is 23?
Number next to element name is the mass #
To figure out the number of neutrons, subtract the number of protons from the mass number.
Atomic number=
Number of protons
How many neutrons does Carbon-13 have?
7
The average atomic mass of gold with the 50% being gold-197 and 50% being gold-198.
What is 197.5 amu?
The number of electrons in a Polonium atom?
84 electrons
How many neutrons are in a K-39 isotope? (Hint- it's in your green notebook)
20 neutrons
How many protons are in a Cu-63 isotope?
29
How do you find the number of neutrons?
Subtract the atomic number (number of protons) from the mass number (total number of protons and neutrons)
Calculate the average atomic mass of iridium using the following data for two iridium isotopes. Isotope mass (u) relative abundance
Ir-191 191.0 37.58%
Ir-193 193.0 62.42%
What is 192.25 amu?
How do you find the number of electrons
Start with the atomic number of the element, which tells you the number of protons. The number of electrons equals the atomic number.
The correct name for an atom with 15 protons 15 electrons and 20 neutrons
What is Phosphorus-35?
Bonus: What does 14 stand for in the isotope carbon-14?
Mass number
BONUS: Who is known as the father of the periodic table?
Dmitry Mendeleev
The average atomic mass is
Calculate the average atomic mass of magnesium using the following data for three magnesium isotopes. Isotope mass (u) relative abundance
Atomic Mass Relative Abundance
Mg-24 23.9850 78.99
Mg-25 24.9858 10.00
Mg-26 25.9826 11.01
What is 24.31 amu?
The number of electrons in a Silver-109 atom
What is 47 electrons?
A magnesium atom has 12 protons and 15 neutrons, what is its isotope name?
Magnesium-27